Portable green energy out of the blue: hydrogel-based energy conversion devices
Abstract
To alleviate the escalating global demands for electricity with a low carbon footprint, we can resort to a green energy source that is conveyed by tiny temperature or moisture gradients. A tremendous source of low-grade energy scatters around us and remains unutilized, which is why thermoelectric and hydrovoltaic devices were invented. Our review focuses on a growing trend of implementing hydrogel-based ionic thermoelectric systems and hydrovoltaic devices as they hold the promise of electric outputs that are several times higher than conventional solid-state inorganic counterparts. This is due to the molecular-level tailorable features of hydrogel polymers and their interactions with water and other functional additives, which provide an ideal platform for low-grade heat and water energy harvesting from fundamental and practical perspectives. This review is divided into three sections. We present working principles, engineering concepts, state-of-art designs, and urgent challenges for hydrogel-based (i) ionic thermoelectric systems; (ii) hydrovoltaic devices; and (iii) their hybrids.
Keywords
INTRODUCTION
Two of the biggest challenges of the 21st century at the global level are the energy crisis and climate change issues[1]. To address the increasing worldwide demand for electricity without added carbon emission, energy recovery systems that capitalize on the low-grade energy in terms of low-grade heat or water for direct electricity production offer a promising strategy, especially of which low-grade heat and water (e.g., moisture, droplets) share the same significant advantages of omnipresence and abundance in our living environment. Envision this scenario: the low-grade waste heat ejected by air conditioners is emitted to the outdoors; meanwhile, the indoor humidity is reduced by running air conditioners compared with humid outdoors in the places like Singapore, Hong Kong, New Orleans, etc. Thus, temperature and moisture gradients are built across indoor and outdoor environments and offer a great opportunity for electricity production [Figure 1]. Likewise, our bodies generate a similar temperature gradient or moisture gradient towards the surrounding environment, making easy access to such energy sources with the prospect of power generation.
Figure 1. Two examples of low-grade energy of moisture and low-grade heat co-existing in our living environment. Body heat and moisture from breathing, sweating, cutaneous transpiration, etc., can be harnessed for power generation based on hydrogel materials. Exhaust heat from air conditioners and relative humidity differences between indoor and outdoor environments can also be utilized to produce electricity through hydrogel materials. (Ionic species at oxidized state and reduced state are expressed as Ox and Red, respectively).
Any kind of gradient is equivalent to a driving force that could do work in the system. Conventional electrochemical energy conversion systems have been developed with proven utility and ability to harvest the scattered energy in the environment. This provides us with significant experience in paring electrodes, electrolytes, separators, etc., to optimize these systems from the mechanism, design, and energy conversion efficiency. Particularly, a small difference in temperature or moisture can lead to a reaction entropy change or chemical potential change in an electrochemical system[2,3]. Access to such gradient sources is very appealing for extensive energy recovery and portable power supply. With the aid of gelation chemistry to build a quasi-solid-state device without moving fluids, there is a growing trend of using hydrogel materials to upgrade electric outputs in new ionic thermoelectric systems (i-TEs) and hydrovoltaic devices [Figure 2].
Figure 2. The scope of the present review including hydrogel-based ionic thermoelectric systems, hydrovoltaic devices, and their hybrids.
Hydrogel is a three-dimensional (3D) network of hydrophilic polymers from cross-linked individual polymer chains, which can swell and hold large amounts of water while maintaining a defined structure[4]. Taking advantage of multitudinous and tunable molecular building blocks and rational integration with functional additives such as redox couples, salts, ligands, and secondary polymers [Figure 3A], not only can hydrogels enable water absorption and retention properties and desirable features of transport of mass and ion but also provide large surface areas for active sites and abundant pendant groups for versatile function[5]. These properties underpin their applications in i-TEs and hydrovoltaic devices, where hydrogels have been used as local concentration regulators, polyelectrolytes, transport media, separators, ion channels, surface functional layers, and many more [Figure 3B][6-10]. Despite microcosmic pictures of ion transport in bulk hydrogel or interfacial redox processes remaining to be explored, they are important for us to obtain fundamental insights and optimize macroscopic properties and performance. Generally, hydrogel-based i-TEs and hydrovoltaic devices are operated separately, whereas, as the heat gain (or loss) and corresponding temperature regulations are naturally correlated with the events of water in hydrogels (e.g., condensation, convection, evaporation), the hydrogel component in the one (hydrovoltaic device) can work jointly with the other (i-TE), and vice versa for maintaining the temperature gradient or enhancing water evaporation[10,11] Such synergies between temperature gradient and transpiration induced charge concentration at the electrode interface may be exploited for increasing output voltage [Figure 2].
Figure 3. (A) Hydrogel network and functional additives including redox couples, salt ions, and additive polymers. (B) General routes of utilizing gradient energy with the aid of hydrogel materials. (B1) Phase-change hydrogel serves as a local concentration regulator to suppress or promote redox reactions at the electrode and electrolyte interfaces. (B2) Either dry polyelectrolyte adsorbing water from moist air or wet polyelectrolyte being thermally activated can release charged mobile ions to establish a concentration gradient across the hydrogel. (B3) Introduction of functional additives into the hydrogel can selectively localize anions but facilitate the ions with the opposite charge, consequently building the concentration gradient. (B4) Hydrogel made of nano/microchannels (or designed physical constraints) works as an ion-selective membrane. In (B1-B4) the hydrogel performs in different length scales (i.e., at the interface or in bulk).
In this review, we summarize the fundamental mechanisms and engineering concepts related to the development of hydrogel-based i-TEs, hydrovoltaic devices, and their hybrids. We highlight the similarities in working principles between i-TEs and hydrovoltaic devices and the opportunities for the discharge modes of the former to be extended and adopted by the latter to improve power output. In each session, we start with working mechanisms, then present recent progress in how hydrogel materials were integrated with those energy conversion systems, and conclude with remaining challenges involving unknown mechanisms, future practical applications, and perspectives.
IONIC THERMOELECTRIC SYSTEMS USING HYDROGEL MATERIALS
Abundant sources of waste heat exist in many industries and daily life due to limitations of current power generation technologies; approximately 60% is in the form of low-grade heat (< 100 °C)[12]. Especially considering the environmental energy capture, the applicable temperature window is further narrowed down to ~15 °C (e.g., the human body of 37 °C to the surroundings of 22 °C). Conventional solid-state thermoelectric materials (TEs), including semiconductors and conducting polymers, were expected to be a prioritized solution to regenerate energy from waste heat and supply electric power through asymmetric electron (or hole) distribution and transport of the generated charge carriers in n-type (or p-type) TEs driven by a temperature gradient [Figure 4A]. Because of the small thermopower of approximately
Figure 4. Working mechanism of TE, TGC and TDC. (A) A p-type TE with continuous hole flow driven by a temperature gradient. α is the thermopower, ΔV and ΔT are the potential difference and temperature difference between the hot and cold electrodes, respectively. V(TH), V(TL), TH and TL represent the potential and temperature at the hot and cold electrodes, respectively. (B) A p-type TGC with a redox reaction Ox + ne- ↔ Red, producing a continuous current with electrochemical reactions occurring at the hot and cold electrodes. n is the number of electrons transferred in the reaction, F is the faradic constant, Sox and Sred are the molar entropy of the redox species. (C) A p-type TDC with cations diffusing with higher mobility over anions, producing an intermittent current through the thermal charging and electrical discharging cycle.
Among various i-TEs for low-grade heat-to-electricity conversion, two technical routes have drawn extensive attention, namely, thermogalvanic cells (TGCs) and thermo-diffusion capacitors (TDCs). TGCs have a relatively long history which dates back to the late 1970s when Burrow[19] and Quickenden[20,21] proposed liquid-based alternatives to solid TEs. A typical TGC works under a thermal gradient, with redox reactions occurring at two electrodes to produce a steady current through the external circuit [Figure 4B]. TGCs, leveraging temperature-dependent redox reactions on spatial or time scales, have inspired several auspicious derivatives, such as thermal regenerative cycles (TRECs)[22-28], thermal regenerative ammonia-based batteries (TRABs)[29,30], CO2-induced pH-sensitive thermally regenerative cells (pH-TRCs)[31,32], direct thermal charging cells (DTCCs)[33,34], and more. In contrast, a typical TDC converts heat to electric power based on thermal-induced ion diffusion and is operated like a (super)capacitor to transduce an ion concentration gradient in the electrolyte to the electron flow through the external circuit [Figure 4C]. Both TGCs and TDCs containing redox couples or salt ions fundamentally differ from TEs, in which electrons and holes can directly pass through the electrodes [Figure 4A]. Interfacial reactions (for TGCs) or ions accumulation (for TDCs) occurring at a very small distance from electrode surfaces (within tens of nanometers that are deduced from EDLs) dominate the thermoelectric response in terms of magnitude and speed[35]. Therefore, i-TEs are required to be “electrically thin” to ensure fast kinetics but “thermally thick” to maintain a proper temperature difference.
Various i-TEs have distinct origins of technology; the indicator used to describe thermoelectric performance (e.g., Seebeck coefficient and temperature coefficient) varies from work to work. To be more precise and comparable, the thermopower (α) is defined here with the same expression:
where ΔV and ΔT are the potential difference and temperature difference between the hot and cold electrodes. V(TH), V(TL), TH, and TL represent the potential and temperature on the hot and cold electrodes, respectively. In analogy with solid-state TEs, a system with a positive α will be called “p-type” while the negative α will be called “n-type” in the following discussion.
Biological hydrogels that can convert thermal stimulus into electric signals have been discovered in sharks, and thermoelectric heat pumps exist in wasps[36,37], which suggests the great potential of hydrogel materials to be adopted by i-TEs. A large and growing body of research endeavors has been devoted to hydrogel-based i-TEs, where the interactions among electrodes, ions, and solvent-rich media govern the working mechanisms involving a faradic effect, non-faradic effect, or synergistic effect. As a highly solvent-rich and customizable material, hydrogel exhibits versatile features and exceptional adaptabilities that match well with i-TEs[5,38]. First, benefiting from gelation chemistry, hydrogels can be well integrated into electrodes, polyelectrolytes (with redox couples or non-redox active ionic species), ion channels, and more. Second, a solvent-rich environment permits sufficient ion dissociation and ion transport[5]. Third, the polymer matrix minimizes convective heat transfer in the electrolytes to maintain the temperature gradient for long-term operation. Moreover, an additional benefit for hydrogel-based i-TEs is the ease of serial connections of n-p units to increase the voltage and current output[39]. Other general advantages of hydrogel materials, such as softness and stretchability[8,40,41], self-healing[42], etc., have also been integrated into a variety of i-TEs.
Hydrogels in thermogalvanic cells
In TGCs, a thin layer of electrolyte containing redox couples is sandwiched by hot and cold electrodes [Figure 4B]. The reaction entropy of the redox couple is sensitive to the local temperature. Ions are oxidized or reduced at electrolyte-electrode interfaces to produce electricity continuously. For a redox reaction
| Redox reaction | Thermopower (mV K-1) | Type |
| Fe(CN)63- + e- ↔ Fe(CN)64- | 1.4 | P |
| Fe3+ + e- ↔ Fe2+ | -1.5 to -0.2 | N |
| I3- + 2e- ↔ 3I- | -0.9 to -0.5 | N |
| V3+ + e- ↔ V2+ | -1.7 | N |
| Co(bpy)33+ + e- ↔ Co(bpy)32+ | -1.2 | N |
Thermo-sensitive hydrogel as a local concentration regulator in TGCs
Regulating local concentrations of redox ions (CRed/COx) by using thermo-sensitive (or phase-transition) hydrogels is able to increase α [Figure 3B1], where CRed and COx is the concentration of ionic species at the oxidized state and reduced state, respectively. In 2016, Zhou et al. proposed the concept of thermo-sensitive supramolecular enhanced α of I-/I3- in TGCs using α-Cyclodextrin (α-CD)[50]. The selective host-guest interaction of α-CD and I3- can capture redox-active I3- at a low temperature, thus increasing the CRed/COx and shifting the equilibrium toward the oxidation at the cold electrode, leading to an increased α from -0.86 to
Figure 5. (A) Schematic of TGCs containing only I-/I3- (n-type) and pNIPAm/(I-/I3-) (p-type). (B) The phase change of pNIPAm nanogels and the redox reaction of I-/I3-. (C) Concentration change ratio of I- and I3- at different temperatures. (D) Voc as a function of temperature difference with and without pNIPAm nanogels in the I-/I3- electrolyte. (E) I-V curves and corresponding power at
Hydrogel-enabled p-n TGC series
On the system level, the p-n series connection of TGCs (similar to traditional TE panels) is a straightforward approach to achieving pragmatically usable and large electric outputs and real wearable devices. However, traditional aqueous electrolytes restrict the large-scale integration and packing of TGC units[54]. As the quasi-solid electrolyte has been successfully applied to similar electrochemical energy systems, such as lithium-ion batteries[5,55], it reveals an alternative of polyelectrolyte for the p-n series connection of TGCs. Explicitly, hydrogel materials, e.g., poly(vinyl alcohol) (PVA)[39], polyurethane[52], cellulose[53,56], gelatin[18], and polyacrylamide (PAm)[57], have been tested as the quasi-solid matrix for series packing of TGCs. In 2016, Yang et al. reported a Voc of ~0.7 V and a short-circuit current (Isc) of
Figure 6. (A) 59 pairs of p-type PVA/(Fe(CN)63-/Fe(CN)64-) (PPF) and n-type PVA/(Fe2+/Fe3+) (PCF). The polyelectrolytes were sandwiched between two flexible substrates made of polyimide (PI). (B) Voc as a function of ΔT. (C) I-V curve and the corresponding power density of one pair of p-n series. (D) Voc and Isc of 59 pairs of p-n series by using body heat at an ambient temperature of 5 °C.
Hydrogels in thermo-diffusion capacitors
The Soret effect that ions with opposite charges diffuse at different mobilities in response to a temperature gradient has been ignored because of its small contribution to the overall thermopower for most liquid-based i-TEs. It implies an opportunity to maximize the thermopower by manipulating ion mobilities in hydrogel materials, which can pose either chemical constraints like chelation or physical constraints such as coulombic attraction/repulsion to ionic species [Figure 3B]. In the last decade, a complementary i-TE (i.e., TDC) that capitalizes on distinct ion diffusions of anions and cations has attracted increasing attention. TDCs exhibit an up-to-date record of 43.8 mV K-1, and the value is more than two orders higher than that of TEs[59]. Figure 4C shows the working mechanism of TDCs. Under a thermal gradient, the cations and anions with different mobilities (absolute values or directions) cause an electric field across the system. The thermopower of TDCs is
Hydrogel as ion dissociable polyelectrolyte in TDCs
Hydrogel is ideal as a polyelectrolyte because it provides dissociable ions and a low-viscous background for mass and ion transport and offers sufficient and versatile pendant groups for regulating ion behaviors [Figure 3B2]. A typical example is a poly(sodium 4-styrenesulfonate) (PSSNa) hydrogel, where Na+ cations are diffused against the fixed negatively charged PSS- backbones at a thermal gradient, exhibiting an α of
Figure 7. (A) Schematic of 4-stage charging-discharging cycle of TDC made of NaOH-PEO electrolyte and Au/CNT electrodes at a ΔT = 16 K (Rload = 100 kΩ). (B) Voc of NaO-[CH2-CH2-O]n-Na TDC during charging and discharging by applying a periodic heating source. (C) The charges being transferred during charging (black squares) and discharging (red circles) at different ΔT (Rload = 100 kΩ). (D) Schematic of a TDC made of PSSH polyelectrolyte and PANI electrodes. (E) Redox reactions of PANI during charging and discharging processes (switching between emeraldine salt and leucoemeraldine base). (F) α and electrical conductivity of PSSH TDC at various humidities and measured at different configurations, i.e., in-plane and out-of-plane. (G) 3-stage charging-discharging cycle of PSSH TDC at a ΔT = 5.3 K (Rload = 1 kΩ). (A-C) Reprinted with permission Ref.[17]. Copyright 2016, The Royal Society of Chemistry. (D-G) Reprinted with permission Ref.[68]. Copyright 2016, WILEY-VCH.
Besides inert electrodes (e.g., carbon, gold, and platinum), redox-active electrodes, such as polyaniline (PANI) conducting polymers, were incorporated to transduce the gradient energy of ions in the polyelectrolyte to the PANI electrode via electrochemical reactions. Particularly, the PANI were thermally charged to an oxidation state (leucoemeraldine base at high temperatures) or reduction state (emeraldine salt at low temperatures) [Figure 7D and E]. Additionally, the PANI deposited on highly porous flower-shaped graphene structures entangled by tubular CNTs (1200 F m-2) were assembled with poly(4-styrenesulfonic acid) (PSSH) polyelectrolytes, where an α of 8 mV K-1 at a relative humidity (RH) of 70% was obtained [Figure 7F][68]. Compared with inert electrodes, these redox-active electrodes held the voltage even after the temperature gradient was removed [Figure 7G]. Moreover, it is worth noting that the RH greatly shifted the measured thermopower from 5 mV K-1 (RH = 30%) to 8 mV K-1 (RH = 70%) when the sample was tested with in-plane configuration, implying an underlying water transport related mechanism when a large surface area of hydrogel polyelectrolyte was exposed to the ambient air[69,70].
Hydrogel as an intermediate to regulate ion transport in TDCs
Aside from directly dissolving ions from polymer backbones, a hydrogel can also serve as a medium to house ionic species (i.e., cations and anions), inside which other polymer additives can modify the mobilities of cations and anions to generate very high thermopowers [Figure 3B3]. As an example, the double-network PAm-alginate hydrogel was carefully selected first for its water content of about 86%, which allows the ionic liquid of EmimBF4 to be used as the electrolyte and be dissociated sufficiently; second, the alginate endowed with substantial carboxyl functional groups can localize Emim+ ions; third, the PEG was added to further impede the ion transport of Emim+ through hydrogen bonding [Figure 8A][8]. As a result, a large disparity of the mobility between Emim+ and BF4- was created, and therefore a very high thermal voltage was obtained when a temperature gradient was built, corresponding to an α of 19.32 mV K-1. The distinct mobilities of cations and anions [Figure 8B and C] at different temperatures were, for the first time, characterized by 2D-diffusion-ordered spectroscopy (DOSY) nuclear magnetic resonance (NMR), allowing a quantitative analysis of ion transport inside the hydrogel (or polyelectrolyte) rather than intuitive assumptions. The voltage profile during charging-discharging of a PAm-alginate/EmimBF4/PEG is shown in Figure 8D and E, and a maximum power density of 0.31 μW cm-2 was achieved at a ΔT of 3.1 K. Another example of using polymer additives to regulate ion transport is PEG in poly(vinylidene fluoride-co-hexa-fluoropropylene) (PVDF-HFP) with EmimTFSI ionic liquid [Figure 9A][71]. In pristine PVDF-HFP/EmimTFSI gel, fluorine atoms on the polymer matrix serving as the strong electron-withdrawing groups were likely to increase the local molecular order and enhance the thermo-diffusion of anions, contributing to the α of -4 mV K-1 for PVDF-HFP/EmimTFSI. After adding PEG into PVDF-HFP/EmimTFSI gel, the oxygen atoms in the PEG molecules were likely to enhance the thermo-diffusion of cations over anions, thus tuning the α from -4 to 14 mV K-1 [Figure 9B and C]. In addition, a PVDF-HFP/EmimDCA/NaDCA TDC was reported to reach a large α from 25.5 to 43.8 mV K-1 by using 0.5 mol% sodium dicyanamide (NaDCA) additive to EmimDCA [Figure 9E-G][59]. An increased mobility difference between cations and anions originates from Na+ cation doping. The p-type nature of PVDF-HFP/EmimDCA indicates DCA- moves slower than Emim+ in the gel matrix, corresponding to an α of 25.5 mV K-1. After adding NaDCA, the relatively strong Coulomb attraction between Na+ and DCA- ions hindered the diffusion of DCA- ions, thus enlarging the mobility difference. In contrast, Cl- anion doping (adding EmimCl) had the opposite effect, which hindered the diffusion of Emim+ and decreased the α from 25.5 to 22.0 mV K-1. Moreover, other dopants like lithium chloride (LiCl) and magnesium chloride (MgCl2) barely changed α as they slowed down both cations and anions in the gel matrix, where no further mobility difference between cations and anions could be developed [Figure 9G]. As a result, PVDF-HFP/EmimDCA/NaDCA outperformed other systems and exhibited a maximum power density of 11.2 mW m-2 with a Rload of 1 kΩ at a ΔT of 0.5 K
Figure 8. (A) Schematic of Pam-alginate/EmimBF4/PEG TDC. (B) Diffusion coefficients of Emim+ and BF4- as a function of PEG concentration. (C) Diffusion coefficients of Emim+ and BF4- as a function of temperature. (D) 4-stage charging-discharging cycle of Pam-alginate/EmimBF4/PEG TDC at a ΔT = 3.4 K (Rload = 1 kΩ). (E) I-V curve and the corresponding power density of Pam-alginate/EmimBF4/PEG TDC at a ΔT = 3.1 K. Reprinted with permission Ref.[8]. Copyright 2021, Elsevier.
Figure 9. (A) Schematic of PVDF-HFP/EmimTFSI TDC, with and without PEG addition. (B) Voc of EmimTFSI, PVDF-HFP/EmimTFSI, and PVDF-HFP/EmimTFSI/PEG as a function of ΔT. (C) Diffusion coefficient of Emim+ and TFSI- with different PEG content. (D) Voc of 18 pairs p-type PVDF-HFP/EmimTFSI/PEG and n-type PVDF-HFP/EmimTFSI connected in series at different ΔT. (E) Chemical structures of EmimDCA, PVDF-HFP, and NaDCA. (F) Ionic conductivity and α of PVDF-HFP/EmimDCA/NaDCA TDC as a function of Na+ doping. (G) Ionic conductivity and α of PVDF-HFP/EmimDCA with different dopants at the concentration of 0.5 mol% with respect to EmimDCA. (H) The 4-stage charging-discharge cycle of PVDF-HFP/EmimDCA/NaDCA at a ΔT = 0.5 K (Rload = 5 kΩ). (I) Voltage during stage II with different Rload. (J) Average power density as a function of Rload with and without NaDCA. (A-D) Reprinted with permission Ref.[71]. Copyright 2019, The Author(s), Nature publishing group. (E-J) Reprinted with permission Ref.[59]. Copyright 2022, Wiley-VCH.
Hydrogel as an ion channel in TDCs
Instead of regulating ion transport inside a homogeneous medium, the hydrogel can be fabricated with a physical channel structure, further improving the capability to localize ions or promote ion transport [Figure 3B4]. Kim et al. designed a Cl- channel by using poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) hydrogel containing cupric chloride (CuCl2) as a functional additive[72]; there was strong metal binding between Cu2+ and PSS polymer chains [Figure 10A]. In the PEDOT:PSS dispersion, the hydrophilic PSS polyanion shell prevented the agglomeration of PEDOT:PSS clusters [Figure 10B]. After adding CuCl2, the strong ionic cross-linking of Cu2+ and PSS shells pulled the clusters together and left a free water/anion channel in the wet conductive gel, which can be seen from the time-of-flight secondary ion mass spectrometry (ToF-SIMS) map of the Cl- distribution image [Figure 10C]. This channel permitted the ion transport of Cl- under the temperature gradient, therefore the p-type nature of the pristine PEDOT:PSS was converted to n-type with a negative thermopower of -18.2 mV K-1. A fluorescence image showing the color change in response to local Cl- concentration indicated Cl- transport at the temperature gradient
Figure 10. (A) Schematic of PEDOT:PSS/CuCl2 TDC. (B) Hydrodynamic radius (Rh) of PEDOT:PSS/CuCl2 particles in the diluted solutions. (C) ToF-SIMS map of the Cl- distribution on the cross-section. (D) Cl- fluorescence mapping in heating-cooling cycles of the PEDOT:PSS/CuCl2 TDC under UV lamp and (E) corresponding thermal images. (F) Schematic of PVA/HCl TDCs and H+ channels. (G) Schematic illustration of ions in PVA/HCl TDC at a ΔT. (H) 4-stage charging-discharging cycle of PVA/HCl TDC at a ΔT = 1 K
Hydrogel-enabled p-n TDC series
Hydrogel-based TDCs can be connected and packed to form p-n series to enhance voltage output[71,73]. P-n pairs of PVDF-HFP/PEG/EmimTFSI with an α of 14 and -4 mV K-1 can be either manually assembled or screen-printed [Figure 9D][71]. 18 p-n pairs generated about 1 V at ΔT = 3 K, corresponding to 94% of the sum of individual thermoelectric legs. Whereas 20 p-n pairs made by screen-printing only exhibited the α of
Synergistic effect of thermo-diffusion and redox reaction
Generally, TGCs with thermogalvanic effect provide small thermal voltage but large current, while TDCs with thermo-diffusion effect provide large thermal voltage but small current. To pursue i-TEs with high energy conversion efficiency in terms of both voltage and current, the integration of two systems in one hydrogel-based i-TE was proposed. In a rationally designed i-TE comprising p-type and n-type elements, there is a chance for the effects, i.e., thermogalvanic effect of redox couple and Soret effect of salt ions, to promote but not to cancel each other [Figure 11A-C]. Starting the hypothesis that thermopowers can be added or subtracted, the i-TE hybrid using gelatin that combined the thermogalvanic effect of Fe(CN)64-/Fe(CN)63- (αr < 0, i.e., α > 0) and the Soret effect of KCl (αtd > 0, i.e., α > 0) was reported by Han et al., where TGC and TDC elements mutually reinforced faradic redox reactions and asymmetric ion transport of K+ over Cl- [Figure 11D][18]. A positive thermopower of 17.0 mV K-1 was obtained, and the estimated contribution from each effect is shown in Figure 11E, as 10.2% from gelatin hydrogel (thermo-diffusion of H+ from the ionization of -COOH group), 17.9% from Fe(CN)63-/Fe(CN)64- redox entropy change, 9.7% from thermo-diffusion of K3Fe(CN)6 and K4Fe(CN)6, and 62.2% from thermo-diffusion of KCl. To date, gelatin/(Fe(CN)63-/Fe(CN)64-)/KCl gave the recorded high power density of 0.66 mW m-2 K-2 for one single i-TE. Figure 11F and G show the quasi-continuous charging-discharging process of the system, where the cell was discharged for 10 s and recharged for 3 mins at a ΔT of 8.5 K for 100 cycles. A maximum power density of 0.66 mW m-2 K-2 was achieved. Furthermore, when connected to a Rload of 5,000 Ω, the cell reached a relatively steady stage with the highest energy density of 12.8 J m-2 [Figure 11H and I]. Finally, a Voc of 2 V and peak power of 5 μW were achieved using body heat by connecting 25 unipolar i-TEs at a ΔT of 10 K
Figure 11. Schematic potential profile of (A) gelatin/KCl, (B) gelatin/(Fe(CN)63-/Fe(CN)64-), and (C) gelatin/(Fe(CN)63-/Fe(CN)64-)/KCl i-TEs. (D) Schematic illustration of diffusion, redox reactions, and interaction of various ions in gelatin/(Fe(CN)63-/Fe(CN)64-)/KCl i-TE at a ΔT. (E) The fractional contribution of each effect to α in gelatin/(Fe(CN)63-/Fe(CN)64-)/KCl i-TE. (F) Voc of the i-TE in quasi-continuous charging-discharging at a ΔT = 8.5 K for 100 cycles. (G) Power, voltage, and current during the fifth discharging in (F).
Challenges for hydrogel-based i-TEs
Table 2 summarizes most of the best i-TEs, in which (poly)electrolytes, electrodes, test conditions, thermopower, and power output are documented for a comprehensive comparison. Efforts to improve electric outputs at a low-grade heat range have given many gratifying results over the past decade using hydrogels and their analogs. Attention is now moving to the next stage of real device power, which is still low compared with other miniaturized energy conversion counterparts. Also, the inconsistency in experimental measurements and calculation methods create obstacles to future developments. Concerning experiments, the temperature gradients are very different in terms of value and direction (in-plane and out-of-plane) in various cases. Some very high thermopower outputs were reported with extremely small temperature gradients (≤ 1 K) or were measured at in-plane configurations[40,59,73]. Unfortunately, the inaccuracy of thermopower characterization became obvious when decreasing the temperature difference, as the systematic error may dominate the results. A proper way is to assess the thermopower at both small and moderate temperature differences (e.g., from 1-50 °C). Meanwhile, in-plane measurements in an open environment (without strict encapsulation) could lead to misinterpretation of electric responses because the effect of the solvent may be more pronounced than that of solutes, which is likely to cause the hydrovoltaic phenomena (this will be discussed in the next session). Thus, a reliable encapsulation is essential for accurate measurement of the thermopower as well as preventing water loss for maintaining long-term cyclability. Several encapsulating methods, such as soft substrate sealing[39,52], (vacuum) heat-sealing[8,18], hydrophobic elastomer coating[57], etc., have been applied to hydrogel-based i-TEs so far. Besides, the electrode materials (i.e., inert electrodes of gold, carbon, etc., and redox-active electrodes of Cu, PANI, etc.) primarily dictate the working mechanisms, which should be carefully used and studied[18]. Intrinsic thermoelectric responses stemming from designated redox reactions or latent corrosion of electrode materials should be taken into account when evaluating the overall thermopower of the device, where the latter is detrimental to the long-term stability and reversibility. In short, the measurement conditions need to be clearly stated, and multi-temperature gradients with accurate ΔT are essential for assessing thermopower. Secondly, since TGCs and TDCs abide by different technical origins and both are deviated from solid TEs, a valid and predictable model is required to improve accuracy for the evaluation of a “new” figure of merit, power density, and energy conversion efficiency. So far, the performance of i-TEs has been calculated by various means, so they cannot be fairly compared from work to work. Thirdly, the absence of comprehensive theory hinders the understanding of hydrogel-based i-TEs and further exploits i-TE designs. With increasing ion concertation and the involvement of soft, charged, and confined interfaces (of hydrogel polymer) in i-TE systems, Debye length, ion solvation, and even the local dielectric constant are hard to be predicted using conventional models. There is a lack of awareness of microcosmic interactions among water, hydrogel polymers, and ionic species, especially in non-equilibrium thermal fields and among water-polymer binary systems. The interpretation of thermo-electrochemical phenomena in such systems without in-situ quantitative evidence is equivocal. Particularly for TDCs, although it is generally believed that the distinct ion mobilities of anions and cations and the consequent asymmetric charge distribution on a temperature gradient are the causes of thermopower, little experimental evidence was provided to show the differences in ion mobilities[8], while some other observations indicated this explanation was insufficient[67]. In conclusion, a careful investigation into the mechanism of the hydrogel-based i-TEs is very much needed in the future.
Some of best reported i-TEs
| Ref* | Type | Material (Polymer/(redox couples)/other) | Electrode | Test configuration | ΔT (K) | α (mV K-1) | Power** (mW m-2 K-2) | Energy efficiency (%) |
| TGC TDC hybrid[18] | p | Gelatin | Cu, Cu/Au | Out-of-plane | 8.5 | 1.3 | ||
| p | Gelatin/(Fe(CN)63-/Fe(CN)64-) | 4.8 | ||||||
| p | Gelatin/KCl | 6.7 | ||||||
| p | Gelatin/(Fe(CN)63-/Fe(CN)64-)/KCl | 17 | 0.66 | |||||
| TGC[39] | n | PVA/(Fe3+/Fe2+) | Au/Cr | Out-of-plane | 17.5 | -1.02 | 0.0236 (ΔT = 20 K) | |
| p | PVA/(Fe(CN)63-/Fe(CN)64-) | 1.21 | ||||||
| TGC[70] | p | PAm/(Fe(CN)63-/Fe(CN)64-)/K+, Li+, Br- | Ti | Out-of-plane | 16 | 1.2 | 0.007 (ΔT = 20 K) | |
| TGC[56] | n | Cellulose/(Fe(CN)63-/Fe(CN)64-) | Ni | Out-of-plane | 15 | -1.38 | 0.0622 | |
| TGC[41] | p | AM-co-AMPS/(Fe(CN)63-/Fe(CN)64-)/NaCl | Pt | In-plane | 13 | 1.6 | 0.61 (ΔT = 40 K) | 0.16 |
| TGC[50] | n | (I-/I3-) | Pt wire | / | 34 | -0.86 | 1 × 10-4 | |
| n | α-CD/(I-/I3-) | -1.45 | 0.00129 | 3 × 10-4 | ||||
| n | α-CD/(I-/I3-)/KCl | -1.97 | 0.0147 | 3 × 10-3 | ||||
| TGC[52] | n | (I-/I3-) | Graphite | Out-of-plane | 10 | -0.71 | 0.05 (μW) | |
| p | pNIPAm/(I-/I3-) | 1.91 | 0.35 (μW) | |||||
| TGC[53] | n | (I-/I3-) | Graphite | Out-of-plane | / | -0.71 | 0.16 (μW) (ΔT = 15 K) | |
| n, p | MC/(I-/I3-) | -1.32 to 1.48 | (n-type) 0.18 (μW) (ΔT = 15 K) | |||||
| n, p | MC/(I-/I3-)/KCl | -8.18 to 9.62 | (n-type) 0.12 (p-type) 0.36 (ΔT = 15 K) | |||||
| TDC[17] | p | NaOH-PEO | Au/CNT | Out-of-plane | 5 | 11.1 | 5 × 10-4 | |
| TDC[68] | p | PSSH | PANI/CNT | Out-of-plane, In-plane | 5 | 8 | ||
| TDC[67] | p | PSSNa | Au | In-plane | 4 | 4 | 0.004 | |
| TDC[71] | n | EmimTFSI | Ag, NFC/CNT, Ti/Au | In-plane | 6 | -0.85 | ||
| n | PVDF-HFP/EmimTFSI | -4 | ||||||
| n, p | PVDF-HFP/EmimTFSI/PEG | -4 to 14 | ||||||
| TDC[7] | p | TEMPO-oxidized cellulose membrane/NaOH-PEO | Pt | Out-of-plane | 20 | 24 | ||
| TDC[72] | p | PEDOT:PSS | Au, CNT/Au | In-plane | 4 | 7.5 | ||
| n | PEDOT:PSS/CuCl2 | -18.2 | 0.0188 (ΔT = 4.5 K) | 0.34 | ||||
| TDC[8] | p | PAm-Alginate/LiBF4 | Carbon paper | Out-of-plane | 8 | 1.85 | ||
| p | PAm-Alginate/EmimBF4 | 5.61 | ||||||
| p | PAm-Alginate/EmimBF4/PEG | 19.32 | 0.326 (ΔT = 3.1 K) | |||||
| TDC[66] | p | Nafion | Carbon paint | In-plane | 5 | 4.2 | ||
| p | S-PEEK | 5.5 | ||||||
| n | PVA/NaOH | -1 | ||||||
| p | PVA/H3PO4 | 1.6 | ||||||
| p | PDDAC | 19 | ||||||
| TDC[75] | p | PVDF-HFP/EmimDCA | Ag, Au, Cu | In-plane | 0.5 | 26.1 | 0.00233 (ΔT = 0.6 K) | |
| p | PVDF-HFP/EmimTFSI | 2 | ||||||
| p | PVDF-HFP/BmimBF4 | 6 | ||||||
| p | Agarose/EmimDCA | 11.9 | ||||||
| p | PVA/EmimDCA | 13.5 | ||||||
| p | Gelatin/EmimDCA | 22.1 | ||||||
| p | PVDF/EmimDCA | 23.1 | ||||||
| TDC[40] | p | Polyurethane/EmimDCA | Ag | In-plane | 0.7 | 34.5 | ||
| TDC[59] | p | PVDF-HFP/EmimDCA | Ag | In-plane | 0.35 | 29 | 26.8 (ΔT = 0.5 K) | |
| p | PVDF-HFP/EmimDCA/NaDCA | 43.8 | 44.8 (ΔT = 0.5 K) | |||||
| TDC[73] | p | PVA/HCl | Carbon | Out-of-plane | 1 | 38.20 | ||
| p | PVA/HNO3 | 18.50 | ||||||
| p | PVA/H2SO4 | 12.26 |
HYDROVOLTAIC TECHNOLOGIES USING HYDROGEL MATERIALS
Water empowers the biggest energy reservoir on the Earth as it absorbs the tremendous energy of
Figure 12. (A) Some common water sources can be harnessed with hydrovoltaic media and a schematic illustration of ions dissociation from the hydrogel backbones after imbibing moisture. (B) Three types of hydrovoltaic media and corresponding characteristic electric outputs, top: graphene with moving droplet of ionic solution; middle: graphene oxide; bottom: polyelectrolyte hydrogel.
A number of previous studies have postulated convergence criteria pertaining to ideal media for transduction of a water gradient to electricity, including fast water absorption/desorption kinetics, high density of polar functional groups (or high zeta-potential), large surface area of the materials, and high-speed mass transport. Hydrogel materials answer these demands properly as their 3D network of hydrophilic polymers is endowed with desirable water absorption and retention properties and fast mass and ion transport features. Besides, the abundance of pendant groups on hydrogel polymers offers tailorable charge polarities and provides substantial dissociable ions. Due to the extraordinary versatility of hydrogels, they have been used as polyelectrolytes, functional surface layers for the electrodes, water retention or absorption layers, and even ion-selective channels. In the following section, the recent advances in hydrovoltaic technologies using hydrogel materials are reviewed and discussed. It is worth noting that the operating hydrovoltaic devices are accompanied by exchanging the moisture/water with the surrounding environment, along with the transition of the materials from the dehydrated state to the hydrated state or vice versa. The nanomaterials, e.g., protein nanowires and biological nanofibrous composed of hydrophilic polymers that can swell and retain water, are included in this discussion as one type of derived hydrogel materials.
Moisture-enabled electric generator with hydrogel polyelectrolyte
Many research endeavors have been devoted to the energy transduction from moisture (or humidity) gradient to electricity because the gas-liquid moisture is omnipresent in the air and involved in myriad natural and daily events. The first generation of moisture-enabled electric generators (MEGs) made of graphene or its derivatives was invented by Zhao et al., and a model of diffusion-osmosis was proposed[88]. Asymmetric moisturizing of the MEG equipped with the pendant groups that can provide dissociable H+ enables the energy transduction from moisture gradient to electricity. Particularly, H+ dissociated from oxygen-containing groups of the graphene oxide film during moisturizing, creating a local concentration increase of mobile H+, which consequently diffused along with the reduction of its chemical potential to the dehydrated part (in the absence of mobile ions) and produced the electric signals [Figure 12B]. Though the maximal voltage output approached 1.5 V[81], the MEGs using graphene derivatives generally suffered from the transient voltage output[89].
The hydrogel materials, serving as polyelectrolytes, were introduced to develop the second generation of polymer moisture-enabled electric generators (PMEGs), which can produce stable DC electricity
Figure 13. (A) Scheme of a PMEG comprising a PSSA membrane and two gold electrodes. (B1-B3) The proposed mechanism of the PMEG. Up: The homogeneous distribution of the functional groups. Middle: Incoming moisture ionized the PSSA membrane and released the protons from the polymer chains. Down: The ionized protons migrated to the other side, leaving the negatively charged polymer chains. (C) Voc detected on a PMEG (1 × 1 cm2) at constant moisture. (D) A mist-powered light system where each LED bulb was powered by six flexible PMEG units connected in series. (E) Schematic of a HMEG with a bilayer of polyelectrolyte films (BPF), which enabled spontaneous water adsorption and ion dissociation in moist air. Based on this heterogeneous structure, mobile Cl- and H+ ions diffused in opposite directions. (F) Schematic of a large-scale integration of HMEG units by sequentially aligned stacking strategy and photographs of flexible HMEGs. (G) Plot of voltage output with different serial numbers of HMEG units. Inset is an enlargement of serial numbers ranging from 1 to 140. (H) Stable voltage signals of the integrated device with 10, 40, 70, 110, 150, 270, 560, 1,080, and 1,600 units in series. (A-F) Reprint with permission from Ref.[90]. Copyright 2019 The Royal Society of Chemistry. (E-H) Reprint with permission from Ref.[6]. Copyright 2021, The Author(s), Springer Nature.
Similarly, an ionic hydrogel moisture electric generator (IHMEG) made of PVA-phytic acid (PA)-glycerol hydrogel was developed[91]. A PA with six esterified phosphoric acid groups perfectly served as the proton donor and supplied massive dissociable protons while sufficient water uptake was afforded by hygroscopic glycerol [Figure 14A]. Since the moisture barrier substrate at the bottom of the IHMEG caused asymmetrical moisture absorption across two electrodes [Figure 14B], there was a higher concentration of dissociated H+ at the top, which consequently diffused to the bottom for the reduction of its chemical energy. Notably, the strong water absorption capability achieved by introducing glycerol and weak hydrogen bonds within the IHMEG hydrogel jointly promoted continuous ion dissociation and ion transport. As a result, a single IHMEG unit of 0.25 cm2 attained a stable DC voltage of ~0.8 V (referring to a β of about 1 V per 100% RH) for more than 1,000 h and a DC current of ~9 μA for over 20 h [Figure 14C].
Figure 14. (A) Schematic of an IHMEG device and its functional components. (B) Schematic of the asymmetric moisture stimulus-induced potential in an IHMEG. (C) Voc (red curve) of an IHMEG device over time under an open ambient environment. The ambient relative humidity (blue curve) and temperature (black curve) were synchronously recorded. (D) Left: Schematic of the gradient distribution of ClO4- in gradient-3D-PPy. Middle: Free Li+ ions were ionized by adsorbed water vapor and caused a density gradient of Li+ ions. Right: In the desorption process of water vapor, the recombination of Li+ ions and ClO4- reduced the potential, resulting in a reverse electron flow in the external circuit. (E and F) voltage and current of the g-3D-PPy framework in response to a periodic RH variation (ΔRH = 85%). (A-C) Reprint with permission from Ref.[91]. Copyright 2022, The Authors, Wiley-VCH. (D-F) Reprint with permission from Ref.[92]. Copyright 2016, Wiley-VCH.
Constructing an intrinsic gradient of dissociable ions inside the hydrogel materials is another way to utilize liquid-gas moisture when exposed to moisture uniformly. For instance, hygroscopic polypyrrole (PPy) was used because of its ability to absorb water from ambient air and 3D microporous structures, in which lithium perchlorate (LiClO4) (in solution) was doped through an electrochemical method[92]. To apply a bias voltage of 3 V to a 3D PPy framework, the positive side was oxidized, forming the concentration gradient of ClO4- from the positive electrode (high) to the negative electrode (low). When the entire device was exposed to the moisture uniformly, the ingress of water molecules in 3D PPy led to the breaking of the ionic bond between Li+ and ClO4-, making mobile Li+ and localized ClO4- [Figure 14D]. Since the bottom of 3D PPy framework was heavily doped by LiClO4 with more mobile Li+ generated during moisturizing, Li+ diffused from the high concentration side to the low concentration side, and the voltage output of ~0.06 V and current of 10 μA cm-2 was achieved [Figure 14E and F].
Hydrogel-based surface functional layer for a hydrovoltaic generator
In the aforementioned MEGs, the hydrogel materials work as quasi-solid-state polyelectrolytes, donating the mobile unipolar ions while sufficient water uptake is acquired. Such processes yield long-distance ion diffusion with respect to the concentration gradient. The response time of electric signals could be estimated as follows:
Figure 15. (A) Schematic of an ionic polymer-hydrogel-carbon composite (IPHC) device. (B) Voc of an IPHC and corresponding RH profile in response to moisture flow. (C) Intermittent supply of moisture flows (every 3 s) to an IPHC device and its voltage output. (D) Left: Schematic of an IPHC in the initial dry state. From left to right is carbon paper, Nafion, pNIPAm, and pored carbon paper. Right: in response to moisture flow, the concertation gradient of H2O and H+ is built across the IPHC. (E) Voltage signals recorded by the IPHC corresponding to human breath through nose and mouth. (F) Magnified figure of the red-square region in (E).
Hygroscopic media for hydrovoltaic generators
Beyond artificially supplying moisture or placing the hydrovoltaic device in a high humidity environment, the hygroscopic materials, including biological polymers and the combination of hydrogel with hygroscopic salts, enable the hydrovoltaic alternatives, which are less dependent on a liquid water source and can work spontaneously to capture the moisture from the air. Thus, the operating window in terms of the RH range and scope of application can be extended. For instance, a thin film of hygroscopic protein nanowires gained from the microorganism G. sulfurreducens was reported to exhibit continuous voltage output of 0.4-0.6 V for over 2 months at the RH of 40%-50% [Figure 16B][95]. Such a device abided by the same electricity generation principle as most MEGs, i.e., the transduction of a moisture gradient to a concentration gradient of dissociated charge carriers. As only the protein nanowires at the top surface were able to access and capture the moisture in the air, a vertical moisture gradient was built from the tiny gold electrode at the top to the large gold electrode at the bottom, which was completely insulated from the ambient air [Figure 16A]. The protein nanowires possessed abundant carboxylic groups where the diffusion of free protons against the immobile COO- anionic background was responsible for the observed voltage. It was important to design a proper thickness of protein nanowires where the entire film was within the interfacial vapor-pressure gradient and retained a dynamic equilibrium of water adsorption-desperation exchange at the interface[96]. Therefore, the moisture penetration rate was pretty slow, and the penetration depth was inversely proportional to the film thickness [Figure 16C-E]. The protein nanowires of about 10 µm ensured a stable moisture gradient across the device, and the long-term stability of electric output was preserved. Additionally, various configurations of the device, such as the horizontal configuration with the left electrode exposed to the air, were developed to further verify the correlation between the direction of the moisture gradient and the voltage signals [Figure 16F].
Figure 16. (A) Top: Transmission electron microscope images of the purified protein nanowires. Scale bars, 100 nm. Bottom: diagram of the device. (B) A continuous recording of open-circuit voltage from a device for more than two months. (C) The moisture adsorption ratio (WH2O) in nanowire films is plotted against film thickness (d) at an ambient RH of roughly 50%. (D) Diagram showing a vertical moisture gradient in the nanowire film. (E) ifference in moisture adsorption (ΔWH2O) and output voltage plotted against d. (F) A residual open-circuit voltage of about 0.8 V was measured from a pair of top electrodes in a nanowire device with half of the top surface covered by a glass slide. (G) Schematic of the structure and fabrication process of the AHS. (H) Surface potential distributions of the AHS. (I) Schematic of the wet-dry interface in an AHS after water absorption. (J) Energy conversion pathways in the AHS based on cations adsorption on carbon surface for EDL formation. (K) The correlation between Voc, Isc and the measuring distance (x) between wet-carbon and dry-carbon. (L) Voc of an AHS under constant ambient conditions. (M) Schematic of the moisture-induced self-charging mechanism. (N) Voc and (O) Isc of the device. (A-F) Reprint with permission Ref.[95]. Copyright 2020, The Author(s), Springer Nature. (G-L) Reprint with permission Ref.[97]. Copyright 2022, Wiley-VCH GmbH. (M-O) Reprint with permission Ref.[99]. Copyright 2019, Elsevier.
The hydrogels were also infused with hygroscopic salts to harvest moisture in the air. For instance, the carbon substrate made of carbon black nanoparticles was partially coated with PVA hydrogel containing sodium chloride (NaCl) to fabricate an asymmetric hygroscopic structure (AHS) [Figure 16G][97,98]. By absorbing water from the air, wet-dry asymmetry was developed across the carbon substrate, where the surface potential of wet carbon decreased [Figure 16H]. The EDLs were then formed with Na+ ions adsorbed on the carbon substrate, and such cations aggregated at the contact interface (i.e., wet-dry interface) led to the observed electric outputs [Figure 16I-K]. Especially owing to the high viscosity of this ionic hydrogel, it prevented the interior water from passing across the wet-dry boundary to maintain a long-lasting lateral wet-dry interface, contributing to a Voc of ~0.6 V for over 150 h [Figure 16L]. Furthermore, the hygroscopic hydrogel can serve as the transport media for the charge carriers. A moisture-induced self-charging device (MISD) was fabricated, which consisted of two lateral carbon nanotube electrodes bridged by hydrochloric acid (HCl)/PVA hydrogel with calcium chloride (CaCl2) on one side [Figure 16M][99]. The device’s self-charging process was performed by absorbing water and forming a local CaCl2 solution, which diffused into HCl/PVA hydrogel and caused the diffusion of cations from the CaCl2 side to the other. Interestingly, the authors reported that the transport of cations was selectively facilitated against the anions of Cl- owing to the asymmetric interactions of CaCl2 with HCl/PVA hydrogel. The HCl/PVA played a role in regulating the diffusion coefficients of cations and anions. The cation flux and its accumulation produced a voltage of 0.348 V at RH of 80% and a transient current [Figure 16N and O]. The water uptake of CaCl2 grew to saturation and would lead to the decay of voltage after 1 h.
Selective ion transport media for a hydrovoltaic generator
The evaporation-driven water flow enables another type of hydrovoltaic technology where a classic electrokinetic mechanism of streaming potential is applied[69,100,101]. Such hydrovoltaic devices are equipped with the charged nanochannels or nanopores in the structure and hydrophilic pendant groups on a polymer backbone, where water uptake occurs at the inlet because of either hydrophilic absorption or capillary force, and then water flows through the material and evaporates at the outlet. For example, Figure 17A shows the voltage responses (up to 110 mV) of the hydrovoltaic devices made by several types of biological nanofibrous (NFs), including cellulose, chitin, silk, and lysozyme[100]. The as-prepared NFs were subject to directionally freezing to form the structure with oriented microchannels (1-10 µm) and nanopores
Figure 17. (A) Voc generated by biological generators made of different nanofibrils (NFs). (B) Optical and scanning electron microscope images of aerogel fabricated by directionally freezing. (C) Zeta potentials of biological NF dispersions. (D) Schematic of hydrated channels around and between NFs. (E) Voc variation upon exposure to airflow with different velocities. (F) Structure of the conductive metal-organic framework layered on cellulose nanofibre and the assembled film. (G) Cross-sectional scanning electron microscopy image of the film. Scale bar, 5 µm. (H) Measured zeta potentials of the cellulose nanofibers at different pH values. (I) The streaming potential generated in the hybrid cellulose nanofibre @ conductive metal-organic framework film at different pH (where the NaCl concentration was fixed at 0.01 M) and different salt concentrations (where the pH value was fixed at 10). (J) The measured ionic thermoelectric voltage at a temperature gradient of 5 °C. (K) A schematic of ion distribution and transport in the charged nanochannels and the proposed mechanisms for voltage generation. (A-E) Reprint with permission Ref.[100]. Copyright 2019, Wiley-VCH. (F-K) Reprint with permission Ref.[103]. Copyright 2021, The Royal Society of Chemistry.
Challenges for hydrogel-based hydrovoltaic devices
Although significant enhancements in electric outputs (i.e., voltage, current, or power density) have been achieved in different hydrogel-based hydrovoltaic devices, the understanding of their electricity generation mechanisms is in the infant stage. Specifically, for the charging process (i.e., voltage generation), most hydrovoltaic devices convert the moisture gradient into the concentration gradient of ions in the electrolyte, which shares a similar mechanism to TDCs that are governed by temperature-induced ion transport (e.g., the Soret effect). The asymmetric distribution of cations or anions is responsible for the observed voltage. However, for the discharging process (i.e., current production through external loads), there is still a lack of justified models. The ions in the electrolyte are not able to pass the electrolyte/electrode boundary; that is the reason most TDCs undergo a 4-stage cycle in order to give a discharging current and useful power. Consequently, TDCs were limited to an intermittent working mode (as discussed in Section “INTRODUCTION”). It should be noted that several hydrovoltaic devices worked continuously to produce current and power without going through the cycle. But unfortunately, most studies neglected to explain the reasons. Taking the materials of electrodes (e.g., carbon, gold, and platinum) into consideration[9,90,95,100], faradic redox reactions at the electrode surface could be ruled out. To understand such elusive phenomena, more systematic and careful studies and reliable theories are needed. Moreover, there is likely a trade-off between voltage and current output as most devices were either limited by a small current or a low voltage. The actual power output through the external loads poorly matched the projected value of maximal power density. The valid method of evaluating power density for different systems abiding by various working mechanisms or working modes (i.e., continuous or intermittent) also requires further study.
In practical terms, there is plenty of scope for maximizing electric output and optimizing hydrovoltaic design. Engineering the hydrogel with desirable function groups and controlled nanostructures allows us to tune the water absorption/desorption kinetics and upgrade charge separation capability, which is promising to promote the voltage output, response time, and charge capacity. On the other hand, the continuous and stable electric output in terms of both voltage and current is required to secure a continuous working mode and avoid energy loss in auxiliary rectifier circuits. The gradient energy conveyed by water molecules and ions needs to be fully transferred to the solid electrodes to truly appreciate massive hydrovoltaic energy. Some redox-active electrodes, such as silver/silver chloride, PANI, could be adapted to convert an ion current in liquids to an electron current in the lead wire at the electrode/electrolyte boundary with the occurrence of faradic reactions. Integrating hydrogel polyelectrolytes with conductive hydrogel electrodes is of practical importance in pursuing flexible and wearable hydrovoltaic units.
HYDROGEL-BASED THERMO-WATER COGENERATION
Considering a (photo)thermal energy-water nexus, the spontaneity and universality of water evaporation in hydrogel materials and symbiotic temperature regulating and conduction events imply precious opportunities to integrate thermoelectric and hydrovoltaic units for optimizing energy recovery. Also, the structure conformability of hydrogel materials offers extra merits for pairing up different thermoelectric and hydrovoltaic modules.
For example, a photothermal steam and electricity cogeneration was reported, which had a layer-by-layer structure of PAm hydrogel, polydimethylsiloxane (PDMS) polymer film, bilateral copper oxide/copper foil (CuO/Cu) electrodes, and a cupric sulfate (CuSO4)-PAm hydrogel electrolyte [Figure 18A][10]. Each layer was individually addressed in terms of thickness, composition, and functionality. The outer shell of the PAm hydrogel facilitated a constant supply of water for the whole device, and the inner PAm hydrogel was furnished with a redox-active CuSO4 electrolyte. The nanostructured CuO/Cu electrodes were responsible for the thermogalvanic effect and photothermal effect, by which a temperature gradient across the space between the hot upper CuO/Cu solar absorber and cold bulk water was attained. The PDMS wrap played multiple roles to ensure good contact between the electrodes and the hydrogel electrolyte, prevent the dehydration of the electrolyte, and offer a hydrophobic surface and self-floating capability. As a result, the maximum T of about 8.7 °C and Voc of about 10.3 mV were obtained under one sun irradiation, corresponding to a thermopower of 1.2 mV K-1, and the peak output density reached 1.57 mW m-2
Figure 18. (A) Schematic of the designed integrative photothermal evaporator/thermogalvanic cell. (B) Temperature profiles of the upper and bottom surfaces under chopped one sun illumination and corresponding Voc and Isc (inset: temperature difference between two surfaces). (C) Discharge polarization curves and related power densities of the thermogalvanic cells using Cu foil or CuO/Cu foil as electrodes. (D) Voc of this cell with cycle rotation. (E) Schematic of the structure and working principle of the smart thermogalvanic hydrogel. (F) Temperature variation of the battery at different discharging rates with (red) and without (black) thermogalvanic hydrogel on the surface. (G) Schematic of the experiment setup. Inset shows the cross-sectional view. (A-D) Reprint with permission Ref.[10]. Copyright 2020, Wiley-VCH. (E-G) Reprint with permission Ref.[70]. Copyright 2020, American Chemical Society.
Assembling a hydrovoltaic generator relying on water evaporation and hydrogel-based TGC can bring twofold thermal benefits: escalating the temperature for efficient water evaporation and chilling the cold electrode in TGC. A heat conduction effect enhanced hydrovoltaic power generator (HCEHG) was reported. It was comprised of an i-TE made by gelatin and K4Fe(CN)6/K3Fe(CN)6 and a hydrovoltaic module of porous dual-size Al2O3 (d-Al2O3)[11]. As for the TGC module, in the absence of an artificial temperature control system, a temperature gradient of about 2 K was built from right to left [Figure 19A], where the hot carbon fiber electrode was heated directly by sunlight, and the cold electrode was automatically cooled with the aid of water evaporation in the hydrovoltaic module. A thermal voltage
Figure 19. (A) Schematic of the structure and mechanism of the heat conduction effect enhanced hydrovoltaic power generator (HCEHG) for sustained evaporation electricity output and thermoelectric conversation. (B) Voc the hydrovoltaic module with and without the thermoelectric module. (C) Real-time temperature plots of the surfaces of the dual-size Al2O3 hydrovoltaic module and thermogalvanic module and corresponding temperature difference. The ambient temperature is ~290.4 K. Grey areas indicate that the device is illuminated. (D) Voltage profile of the device with the fully developed oxidation layer under 50% RH as a function of time when the temperature difference was altered. The inset shows the saturated voltage at the corresponding temperature to seek the slope (93 mV K-1). Note it has the opposite sign (-93 mV K-1) based on Equation 1 in this work. (E1-E4) Illustration showing thermoelectric voltage generation and the corresponding potential changes in the hotter and colder sides. Top left: Uniformly distributed water in PANI:PSS under ΔT = 0. Top right: As ΔT > 0, water molecules migrated from the hotter to the colder side. Bottom right: Voltage generation at steady state under ΔT > 0. Bottom left: Water molecules returned to their initial distributed states under ΔT approaching 0. (F) Evans diagram shows the cathodic-reduction reaction (black line), and the anodic oxidation reaction (greenish line) under uniform temperature. The greenish line shifts toward the red (or blue) line with less (or more) water on the electrodes. (A-C) Reprint with permission Ref.[11]. Copyright 2022, The Author(s), Springer Nature. (D-F) Reprint with permission Ref.[104]. Copyright 2021, The Author(s), Springer Nature.
Additionally, the thermo-diffusion of water (not ionic species) in the polyelectrolyte presents another strategy for thermo-hydro-electrochemical power generation in a neat fashion. Zhang et al. reported a device made of hygroscopic polyelectrolyte of polyaniline and polystyrene sulfonate (PANI:PSS) and carbon steel electrodes, from which an ultrahigh thermopower of -93 mV K-1 was achieved [Figure 19D][104]. The PANI:PSS polyelectrolyte absorbed about 15 wt% water from the ambient environment with an RH of around 50%, and the diffusion of water from the hot side to the cold side ensued under a temperature gradient. The asymmetric water distribution caused the shifts of overpotential for carbon steel corrosion
CONCLUSIONS AND OUTLOOK
In this review, the recent progress of i-TEs and hydrovoltaic devices using hydrogel materials has been discussed. Owing to the molecular-level tailorable features of hydrogel polymers and their interactions with water and other functional additives, hydrogels provide an ideal platform for low-grade heat and water energy harvesting from both fundamental and practical perspectives. Versatile hydrogels have been successfully employed in i-TEs, hydrovoltaic devices, and their hybrids as polyelectrolytes, transport media, separators, ion channels, surface functional layers, and more, making big strides forward in the transduction of gradient energy into electricity. Hydrogel-based i-TEs have improved thermopower from a few hundred µV K-1 to tens of mV K-1 at a low-grade heat range, and hydrogel-based hydrovoltaic devices even achieved the hydropower of 1 V per 100% RH and even higher. Such significant electric output is of practical importance for portable energy recovery systems for daily use without the need for auxiliary amplifying and rectifying circuits and capturing scattered heat sources and water sources around us. Nevertheless, there are still many challenges involving unknown working mechanisms that remain to be properly addressed in the future. Overall, current studies of i-TEs and hydrovoltaics are largely based upon empirical studies, but the vast majority of studies have been qualitative. In order to truly appreciate the advances of hydrogel-based gradient energy transduction systems, it is vital to apply more advanced characterization tools and in-situ techniques to observe and understand microcosmic interactions among water, polymers, and ionic species, from which the guidelines of hydrogel selection and optimal design could be deduced. Besides, bulk ion transport, local drifting of ions, interfacial ion accumulation, and redox processes all occur at different length scales. A systematic investigation is needed to build the connection between microscopic characteristic behaviors and macroscopic properties and performances. Moreover, the valid and predictable model of evaluating power density for different systems abiding by various working mechanisms or working modes also requires further study. Specifically, the power density of i-TEs and hydrovoltaic devices basically determines their application and deployment in practice, which, however, is one order lower than some nanofluidic devices (a few W m-2) using gradient energy of salinity gradient[99]. Take hydrogel-based i-TEs as an example, the highest power density that can be achieved by a single module is 0.66 mW m-2 K-2[18]. There is still a gap for a single device to achieve practically useful power. Future efforts could be implemented on developing stimuli-responsive hydrogels for enhanced thermoelectric performance, in which the responsive hydrogel selectively coordinates with redox couple and regulates the local concentration ratio of CRed/COx at different temperatures. This molecular scale responsiveness has the potential to overcome the limited thermopower governed by specific redox couple and corresponding reaction entropy. In addition, other properties of hydrogel materials, including mechanical strength, dimensional stability, water retention capability, anti-freezing capability, and durability under harsh conditions, should be focused on to meet the criteria for real applications. The newly designed hydrogel-based low-grade energy harvesters are expected to open new horizons for extensive applications in the future with their superiorities, including excellent electric outputs, ease of manufacturing, and customizable geometry shape, as well as broad operating range in the ambient environment.
DECLARATIONS
AcknowledgmentsLiu C. and Fang N.X. acknowledge the financial support provided by the Centers for Mechanical Engineering Research and Education at MIT and SUSTech. Feng S.P. and Wang S. acknowledge the financial support provided by the General Research Fund of the Research Grants Council of Hong Kong Special Administrative Region, China.
Author ContributionsInitiated this review: Liu C, Fang NX
Wrote the manuscript: Liu C, Wang S
Provided input for discussions and proofreading: Feng SP
Availability of data and materialsNot applicable.
Financial support and sponsorshipThe Centers for Mechanical Engineering Research and Education at MIT and SUSTech, under Award Numbers 17203520; General Research Fund of the Research Grants Council of Hong Kong Special Administrative Region, China, under Award Numbers 17207422.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Conti J. Annual energy outlook 2013 with projections to 2040. technical report DOE/EIA-0383. Washington, DC, USA: The U.S. Energy Information Administration, 2013.
3. Quickenden TI, Mua Y. A review of power generation in aqueous thermogalvanic cells. J Electrochem Soc 1995;142:3985-94.
5. Guo Y, Bae J, Fang Z, Li P, Zhao F, Yu G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem Rev 2020;120:7642-707.
6. Wang H, Sun Y, He T, et al. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1,000 V output. Nat Nanotechnol 2021;16:811-9.
7. Li T, Zhang X, Lacey SD, et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat Mater 2019;18:608-13.
8. Liu C, Li Q, Wang S, Liu W, Fang NX, Feng S. Ion regulation in double-network hydrogel module with ultrahigh thermopower for low-grade heat harvesting. Nano Energy 2022;92:106738.
9. Liu C, Wang S, Wang X, et al. Hydrovoltaic energy harvesting from moisture flow using an ionic polymer-hydrogel-carbon composite. Energy Environ Sci 2022;15:2489-98.
10. Meng FL, Gao M, Ding T, Yilmaz G, Ong WL, Ho GW. Modular deformable steam electricity cogeneration system with photothermal, water, and electrochemical tunable multilayers. Adv Funct Mater 2020;30:2002867.
11. Li L, Feng S, Bai Y, et al. Enhancing hydrovoltaic power generation through heat conduction effects. Nat Commun 2022;13:1043.
12. Forman C, Muritala IK, Pardemann R, Meyer B. Estimating the global waste heat potential. Renew Sustain Energy Rev 2016;57:1568-79.
13. Bell LE. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008;321:1457-61.
14. He J, Tritt TM. Advances in thermoelectric materials research: looking back and moving forward. Science 2017;357:eaak9997.
15. Cheng C, Dai Y, Yu J, et al. Review of liquid-based systems to recover low-grade waste heat for electrical energy generation. Energy Fuels 2021;35:161-75.
16. Yu B, Duan J, Cong H, et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020;370:342-6.
17. Zhao D, Wang H, Khan ZU, et al. Ionic thermoelectric supercapacitors. Energy Environ Sci 2016;9:1450-7.
18. Han CG, Qian X, Li Q, et al. Giant thermopower of ionic gelatin near room temperature. Science 2020;368:1091-8.
20. Quickenden T, Vernon C. Thermogalvanic conversion of heat to electricity. Solar Energy 1986;36:63-72.
21. Mua Y, Quickenden TI. Power conversion efficiency, electrode separation, and overpotential in the ferricyanide/ferrocyanide thermogalvanic cell. J Electrochem Soc 1996;143:2558-64.
22. Gao C, Lee SW, Yang Y. Thermally regenerative electrochemical cycle for low-grade heat harvesting. ACS Energy Lett 2017;2:2326-34.
23. Ding Y, Guo X, Ramirez-meyers K, et al. Simultaneous energy harvesting and storage
24. Yang Y, Loomis J, Ghasemi H, et al. Membrane-free battery for harvesting low-grade thermal energy. Nano Lett 2014;14:6578-83.
25. Liu Y, Gao C, Sim S, Kim M, Lee SW. Lithium manganese oxide in an aqueous electrochemical system for low-grade thermal energy harvesting. Chem Mater 2019;31:4379-84.
26. Cheng C, Wang S, Tan P, et al. Insights into the thermopower of thermally regenerative electrochemical cycle for low grade heat harvesting. ACS Energy Lett 2021;6:329-36.
27. Sales BB, Burheim OS, Porada S, Presser V, Buisman CJN, Hamelers HVM. Extraction of energy from small thermal differences near room temperature using capacitive membrane technology. Environ Sci Technol Lett 2014;1:356-60.
28. Lee SW, Yang Y, Lee HW, et al. An electrochemical system for efficiently harvesting low-grade heat energy. Nat Commun 2014;5:3942.
29. Zhu X, Rahimi M, Gorski CA, Logan B. A thermally-regenerative ammonia-based flow battery for electrical energy recovery from waste heat. ChemSusChem 2016;9:873-9.
30. Zhang F, Liu J, Yang W, Logan BE. A thermally regenerative ammonia-based battery for efficient harvesting of low-grade thermal energy as electrical power. Energy Environ Sci 2015;8:343-9.
31. Cheng C, Wang S, Wu Y, et al. Thermally regenerative CO2-induced pH-gradient cell for waste-to-energy conversion. ACS Energy Lett 2021;6:3221-7.
32. Cheng C, Wang S, Wu Y, et al. pH-sensitive thermally regenerative cell (pH-TRC) with circulating hydrogen for long discharging time and high-power output. Chem Eng J 2022;449:137772.
33. Wang X, Huang YT, Liu C, et al. Direct thermal charging cell for converting low-grade heat to electricity. Nat Commun 2019;10:4151.
34. Mu K, Mu Y, Wang X, et al. Direct thermal charging cell using nickel hexacyanoferrate (ΙΙ) anode for green recycling of low-grade heat. ACS Energy Lett 2022;7:1146-53.
35. Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications, 2nd ed. New York: John Wiley & Sons; 2001. pp. 12-4. Available from: https://www.wiley.com/en-us/Electrochemical+Methods%3A+Fundamentals+and+Applications%2C+2nd+Edition-p-9780471043720 [Last accessed on 21 March 2023].
36. Ishay JS, Pertsis V, Rave E, Goren A, Bergman DJ. Natural thermoelectric heat pump in social wasps. Phys Rev Lett 2003;90:218102.
37. Brown BR, Hughes ME, Russo C. Thermoelectricity in natural and synthetic hydrogels. Phys Rev E Stat Nonlin Soft Matter Phys 2004;70:031917.
38. Siddique ARM, Mahmud S, Heyst BV. A review of the state of the science on wearable thermoelectric power generators (TEGs) and their existing challenges. Renew Sustain Energy Rev 2017;73:730-44.
39. Yang P, Liu K, Chen Q, et al. Wearable thermocells based on gel electrolytes for the utilization of body heat. Angew Chem Int Ed 2016;55:12050-3.
40. Fang Y, Cheng H, He H, et al. Stretchable and transparent ionogels with high thermoelectric properties. Adv Funct Mater 2020;30:2004699.
41. Lei Z, Gao W, Wu P. Double-network thermocells with extraordinary toughness and boosted power density for continuous heat harvesting. Joule 2021;5:2211-22.
42. Akbar ZA, Jeon J, Jang S. Intrinsically self-healable, stretchable thermoelectric materials with a large ionic Seebeck effect. Energy Environ Sci 2020;13:2915-23.
43. Hu R, Cola BA, Haram N, et al. Harvesting waste thermal energy using a carbon-nanotube-based thermo-electrochemical cell. Nano Lett 2010;10:838-46.
44. Abraham TJ, MacFarlane DR, Pringle JM. Seebeck coefficients in ionic liquids--prospects for thermo-electrochemical cells. Chem Commun 2011;47:6260-2.
45. Liu C, Lee H, Chang YH, Feng SP. The study of electrical conductivity and diffusion behavior of water-based and ferro/ferricyanide-electrolyte-based alumina nanofluids. J Colloid Interface Sci 2016;469:17-24.
46. Poletayev AD, Mckay IS, Chueh WC, Majumdar A. Continuous electrochemical heat engines. Energy Environ Sci 2018;11:2964-71.
47. Kim JH, Lee JH, Palem RR, Suh MS, Lee HH, Kang TJ. Iron (II/III) perchlorate electrolytes for electrochemically harvesting low-grade thermal energy. Sci Rep 2019;9:8706.
48. Buckingham MA, Marken F, Aldous L. The thermoelectrochemistry of the aqueous iron(ii)/iron(iii) redox couple: significance of the anion and pH in thermogalvanic thermal-to-electrical energy conversion. Sustain Energy Fuels 2018;2:2717-26.
49. Taheri A, Macfarlane DR, Pozo-gonzalo C, Pringle JM. Application of a water-soluble cobalt redox couple in free-standing cellulose films for thermal energy harvesting. Electrochim Acta 2019;297:669-75.
50. Zhou H, Yamada T, Kimizuka N. Supramolecular thermo-electrochemical cells: enhanced thermoelectric performance by host-guest complexation and salt-induced crystallization. J Am Chem Soc 2016;138:10502-7.
51. Li X, Liu C, Feng S, Fang NX. Broadband light management with thermochromic hydrogel microparticles for smart windows. Joule 2019;3:290-302.
52. Duan J, Yu B, Liu K, et al. P-N conversion in thermogalvanic cells induced by thermo-sensitive nanogels for body heat harvesting. Nano Energy 2019;57:473-9.
53. Han Y, Zhang J, Hu R, Xu D. High-thermopower polarized electrolytes enabled by methylcellulose for low-grade heat harvesting. Sci Adv 2022;8:eabl5318.
54. Im H, Moon HG, Lee JS, Chung IY, Kang TJ, Kim YH. Flexible thermocells for utilization of body heat. Nano Res 2014;7:443-52.
55. Wang Z, Li H, Tang Z, et al. Hydrogel electrolytes for flexible aqueous energy storage devices. Adv Funct Mater 2018;28:1804560.
56. Jin L, Greene GW, Macfarlane DR, Pringle JM. Redox-active quasi-solid-state electrolytes for thermal energy harvesting. ACS Energy Lett 2016;1:654-8.
57. Ding T, Zhou Y, Wang X, et al. All-soft and stretchable thermogalvanic gel fabric for antideformity body heat harvesting wearable. Adv Energy Mater 2021;11:2102219.
58. Wang XQ, Chan KH, Lu W, et al. Macromolecule conformational shaping for extreme mechanical programming of polymorphic hydrogel fibers. Nat Commun 2022;13:3369.
59. Liu Z, Cheng H, Le Q, et al. Giant thermoelectric properties of ionogels with cationic doping. Adv Energy Mater 2022;12:2200858.
60. Agar JN, Mou CY, Lin JL. Single-ion heat of transport in electrolyte solutions: a hydrodynamic theory. J Phys Chem 1989;93:2079-82.
63. Guthrie G, Wilson JN, Schomaker V. Theory of the thermal diffusion of electrolytes in a clusius column. J Chem Phys 1949;17:310-3.
65. Marcus Y. Effect of ions on the structure of water: structure making and breaking. Chem Rev 2009;109:1346-70.
66. Kim SL, Hsu J, Yu C. Thermoelectric effects in solid-state polyelectrolytes. Org Electron 2018;54:231-6.
67. Wang H, Zhao D, Khan ZU, et al. Ionic thermoelectric figure of merit for charging of supercapacitors. Adv Electron Mater 2017;3:1700013.
68. Kim SL, Lin HT, Yu C. Thermally chargeable solid-state supercapacitor. Adv Energy Mater 2016;6:1600546.
69. Li L, Hao M, Yang X, et al. Sustainable and flexible hydrovoltaic power generator for wearable sensing electronics. Nano Energy 2020;72:104663.
70. Pu S, Liao Y, Chen K, et al. Thermogalvanic hydrogel for synchronous evaporative cooling and low-grade heat energy harvesting. Nano Lett 2020;20:3791-7.
71. Zhao D, Martinelli A, Willfahrt A, et al. Polymer gels with tunable ionic Seebeck coefficient for ultra-sensitive printed thermopiles. Nat Commun 2019;10:1093.
72. Kim B, Hwang JU, Kim E. Chloride transport in conductive polymer films for an n-type thermoelectric platform. Energy Environ Sci 2020;13:859-67.
73. Chen Q, Chen B, Xiao S, et al. Giant thermopower of hydrogen ion enhanced by a strong hydrogen bond system. ACS Appl Mater Interfaces 2022;14:19304-14.
75. Cheng H, He X, Fan Z, Ouyang J. Flexible quasi-solid state ionogels with remarkable seebeck coefficient and high thermoelectric properties. Adv Energy Mater 2019;9:1901085.
76. Stephens GL, Li J, Wild M, et al. An update on Earth’s energy balance in light of the latest global observations. Nat Geosci 2012;5:691-6.
79. Huang Y, Cheng H, Yang C, Yao H, Li C, Qu L. All-region-applicable, continuous power supply of graphene oxide composite. Energy Environ Sci 2019;12:1848-56.
80. Cheng H, Huang Y, Zhao F, et al. Spontaneous power source in ambient air of a well-directionally reduced graphene oxide bulk. Energy Environ Sci 2018;11:2839-45.
81. Huang Y, Cheng H, Yang C, et al. Interface-mediated hygroelectric generator with an output voltage approaching 1.5 volts. Nat Commun 2018;9:4166.
82. Yin J, Li X, Yu J, Zhang Z, Zhou J, Guo W. Generating electricity by moving a droplet of ionic liquid along graphene. Nat Nanotechnol 2014;9:378-83.
84. Cai H, Guo Y, Guo W. Synergistic effect of substrate and ion-containing water in graphene based hydrovoltaic generators. Nano Energy 2021;84:105939.
85. Xue G, Xu Y, Ding T, et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat Nanotechnol 2017;12:317-21.
86. Li J, Liu K, Ding T, Yang P, Duan J, Zhou J. Surface functional modification boosts the output of an evaporation-driven water flow nanogenerator. Nano Energy 2019;58:797-802.
87. Liu K, Ding T, Li J, et al. Thermal-electric nanogenerator based on the electrokinetic effect in porous carbon film. Adv Energy Mater 2018;8:1702481.
88. Zhao F, Cheng H, Zhang Z, Jiang L, Qu L. Direct power generation from a graphene oxide film under moisture. Adv Mater 2015;27:4351-7.
89. Yang C, Huang Y, Cheng H, Jiang L, Qu L. Rollable, stretchable, and reconfigurable graphene hygroelectric generators. Adv Mater 2019;31:e1805705.
90. Xu T, Ding X, Huang Y, et al. An efficient polymer moist-electric generator. Energy Environ Sci 2019;12:972-8.
91. Yang S, Tao X, Chen W, et al. Ionic hydrogel for efficient and scalable moisture-electric generation. Adv Mater 2022;34:2200693.
92. Xue J, Zhao F, Hu C, et al. Vapor-activated power generation on conductive polymer. Adv Funct Mater 2016;26:8784-92.
93. Pope M, Swenberg CE. Electronic processes in organic crystals and polymers. Oxford UK: Oxford University Press, 1999.
94. Ochi S, Kamishima O, Mizusaki J, Kawamura J. Investigation of proton diffusion in Nafion®117 membrane by electrical conductivity and NMR. Solid State Ion 2009;180:580-4.
95. Liu X, Gao H, Ward JE, et al. Power generation from ambient humidity using protein nanowires. Nature 2020;578:550-4.
96. Gouveia RF, Galembeck F. Electrostatic charging of hydrophilic particles due to water adsorption. J Am Chem Soc 2009;131:11381-6.
97. Zhang Y, Guo S, Yu ZG, et al. An asymmetric hygroscopic structure for moisture-driven hygro-ionic electricity generation and storage. Adv Mater 2022;34:e2201228.
98. Zhang Y, Yu Z, Qu H, et al. Self-sustained programmable hygro-electronic interfaces for humidity-regulated hierarchical information encryption and display. Adv Mater 2022:2208081.
99. Luo Z, Liu C, Fan S. A moisture induced self-charging device for energy harvesting and storage. Nano Energy 2019;60:371-6.
100. Li M, Zong L, Yang W, et al. Biological nanofibrous generator for electricity harvest from moist air flow. Adv Funct Mater 2019;29:1901798.
101. Das SS, Pedireddi VM, Bandopadhyay A, Saha P, Chakraborty S. Electrical power generation from wet textile mediated by spontaneous nanoscale evaporation. Nano Lett 2019;19:7191-200.
102. Xiao K, Jiang L, Antonietti M. Ion transport in nanofluidic devices for energy harvesting. Joule 2019;3:2364-80.
103. Zhou S, Qiu Z, Strømme M, Xu C. Solar-driven ionic power generation via a film of nanocellulose @ conductive metal-organic framework. Energy Environ Sci 2021;14:900-5.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Liu C, Wang S, Feng SP, Fang NX. Portable green energy out of the blue: hydrogel-based energy conversion devices . Soft Sci 2023;3:10. http://dx.doi.org/10.20517/ss.2022.32
AMA Style
Liu C, Wang S, Feng SP, Fang NX. Portable green energy out of the blue: hydrogel-based energy conversion devices . Soft Science. 2023; 3(1): 10. http://dx.doi.org/10.20517/ss.2022.32
Chicago/Turabian Style
Liu, Chang, Sijia Wang, Shien-Ping Feng, Nicholas X. Fang. 2023. "Portable green energy out of the blue: hydrogel-based energy conversion devices " Soft Science. 3, no.1: 10. http://dx.doi.org/10.20517/ss.2022.32
ACS Style
Liu, C.; Wang S.; Feng S.P.; Fang NX. Portable green energy out of the blue: hydrogel-based energy conversion devices . Soft. Sci. 2023, 3, 10. http://dx.doi.org/10.20517/ss.2022.32
About This Article
Copyright
Data & Comments
Data

 Cite This Article 34 clicks
Cite This Article 34 clicks



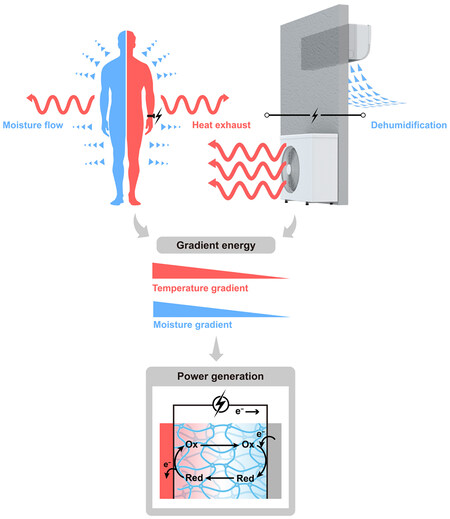
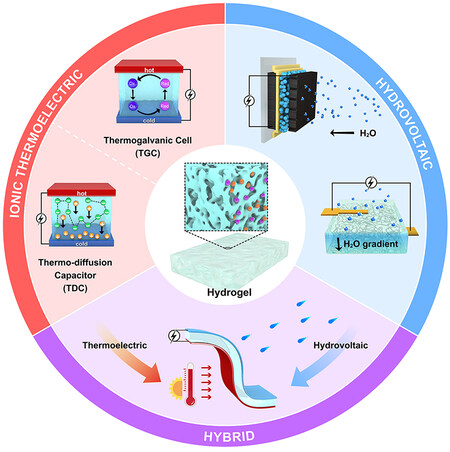
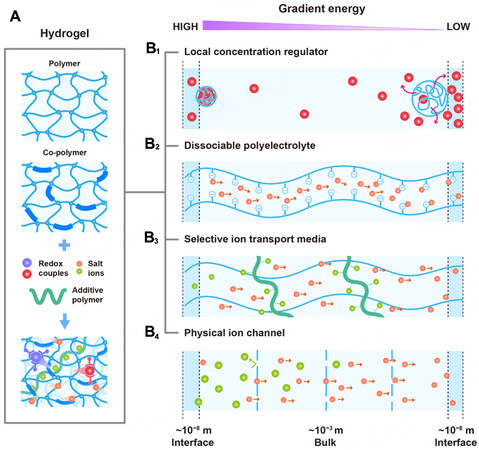
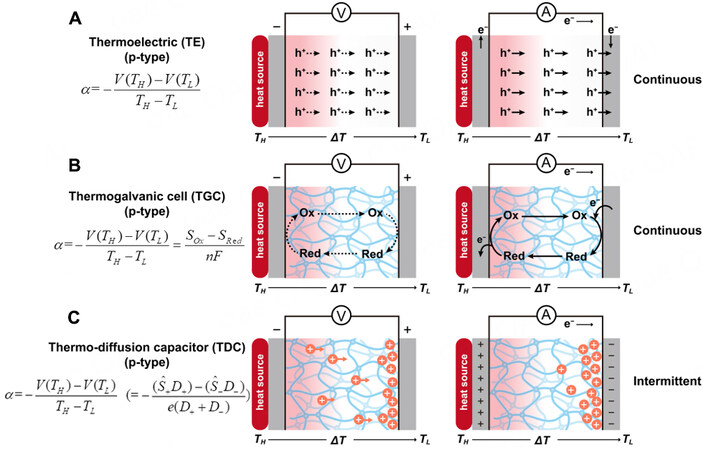
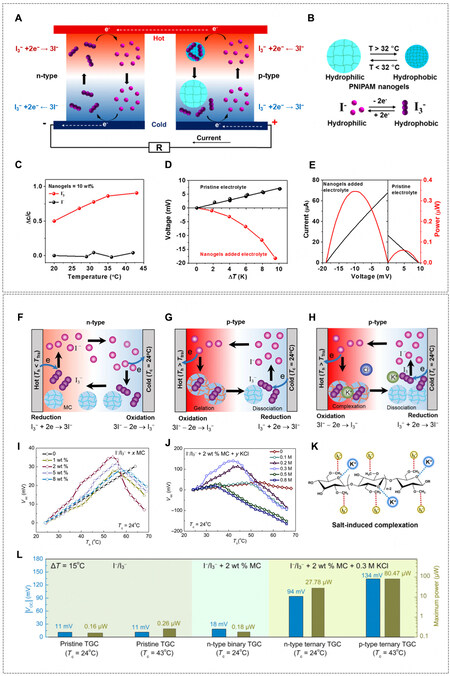
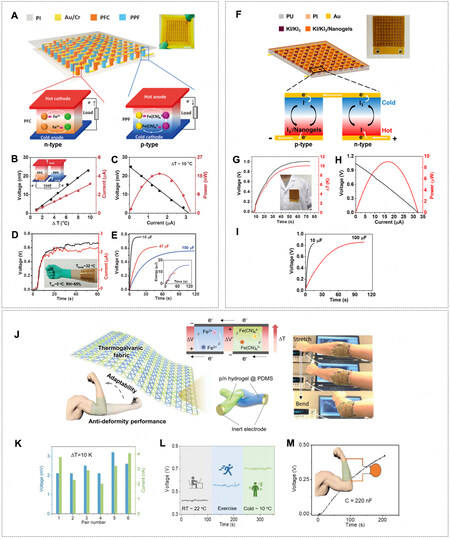
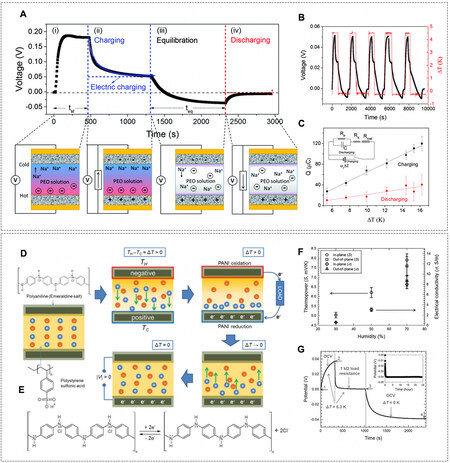
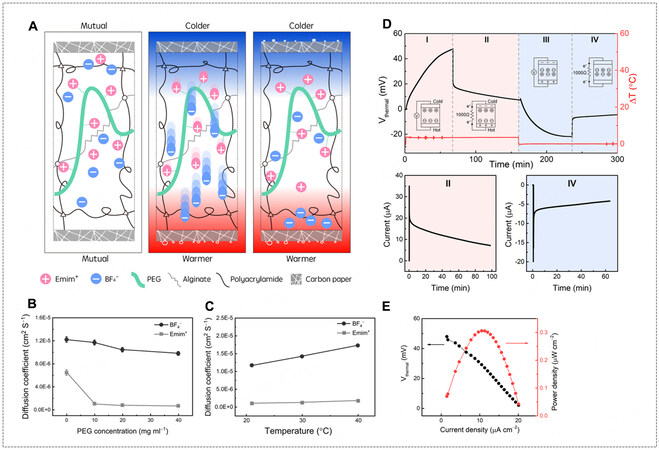
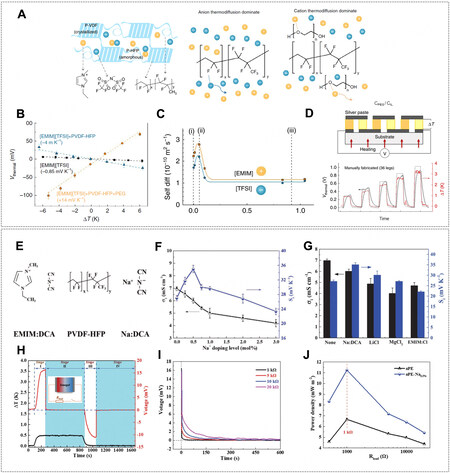
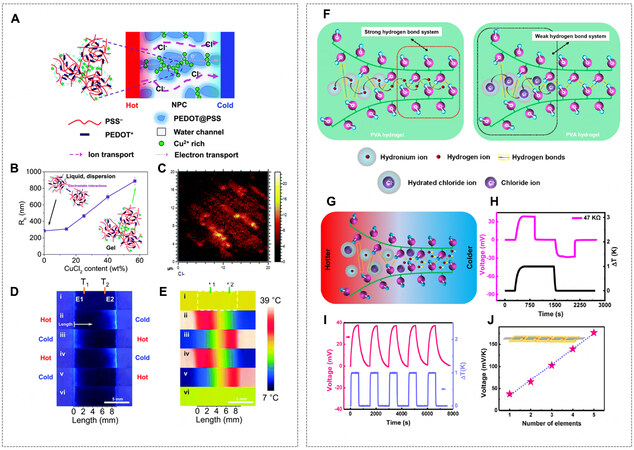
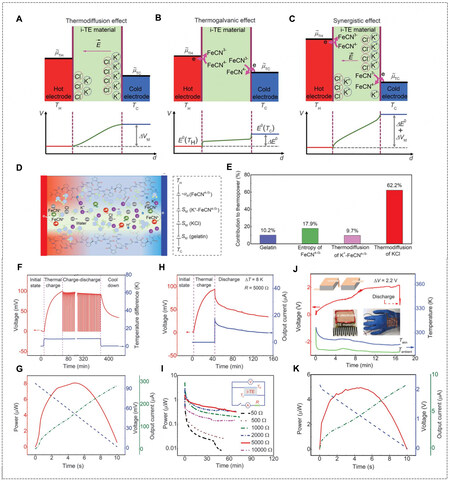
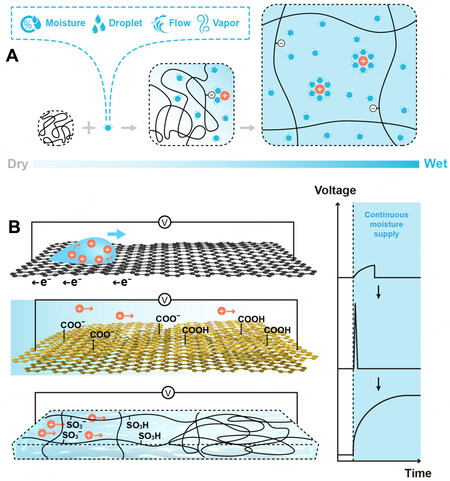
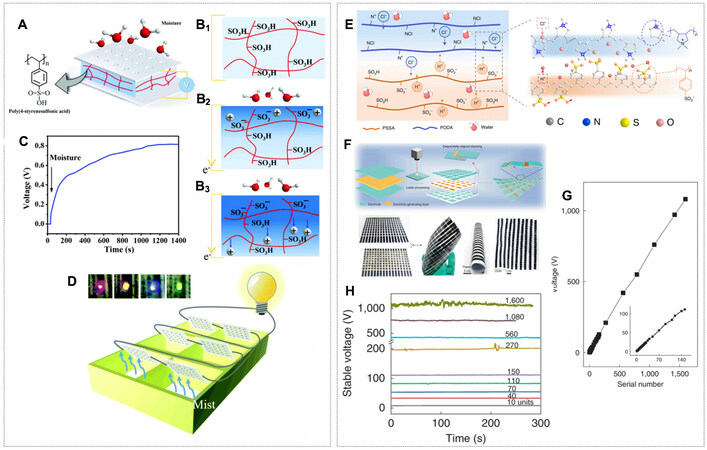
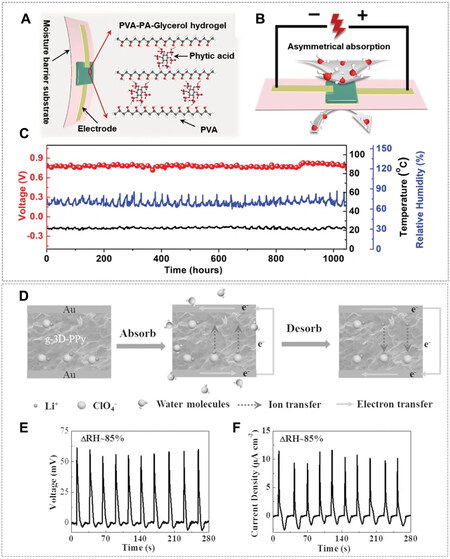
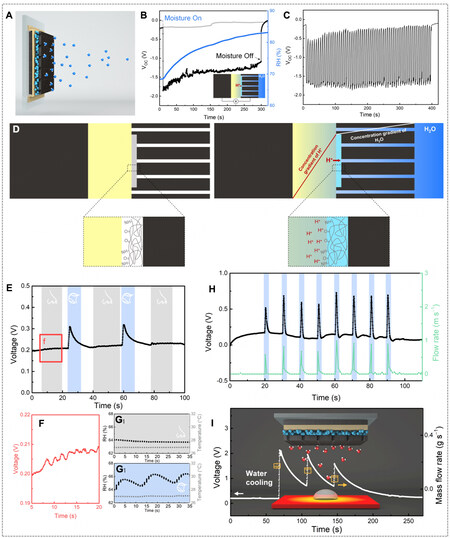
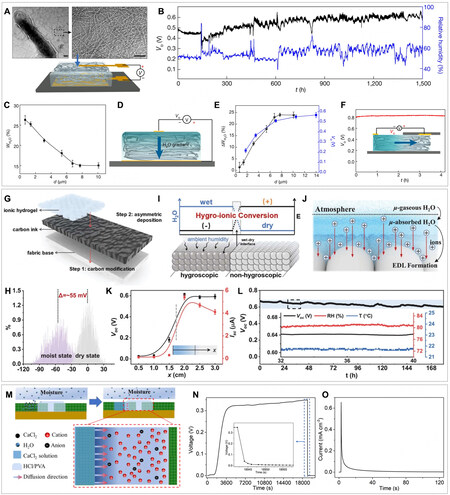
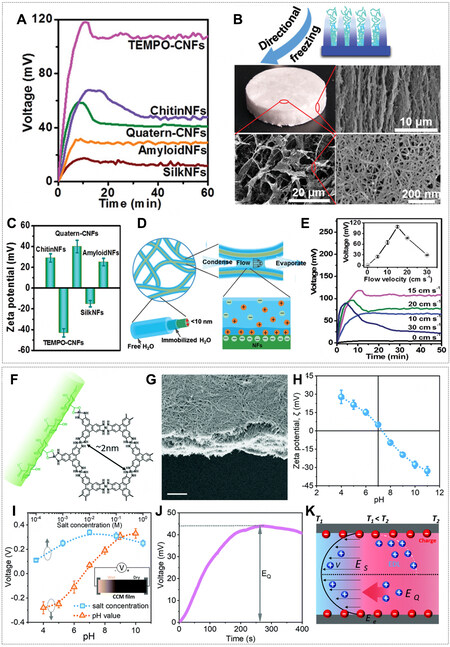
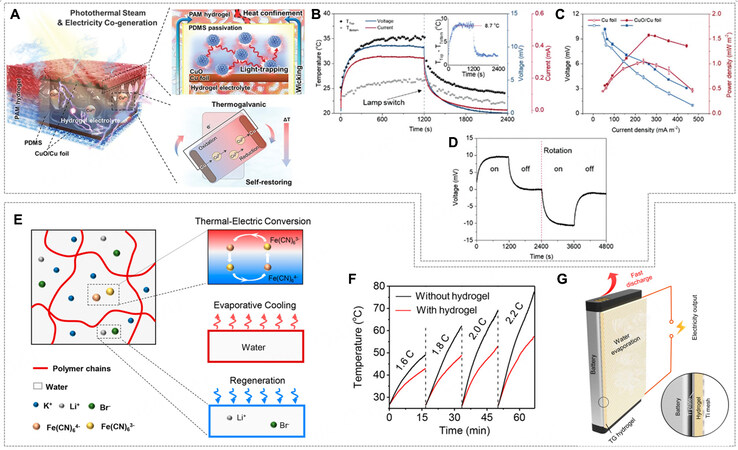
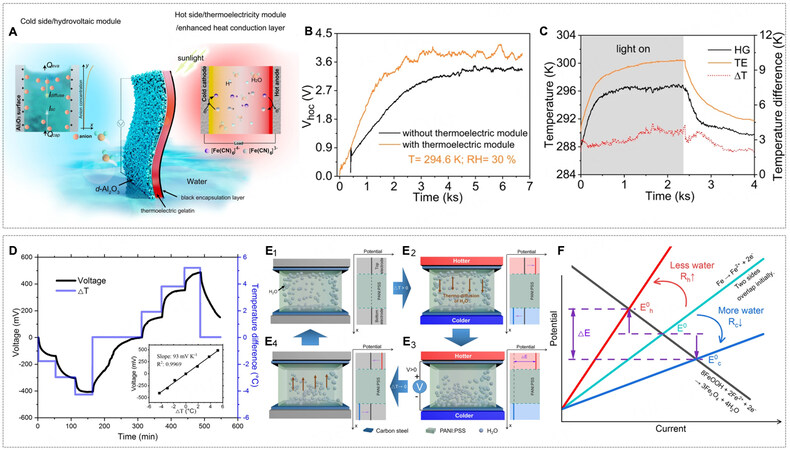










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.