Recent advancements in liquid metal enabled flexible and wearable biosensors
Abstract
Wearable biosensors have demonstrated enormous potential in revolutionizing healthcare by providing real-time fitness tracking, enabling remote patient monitoring, and facilitating early detection of health issues. To better sense vital life signals, researchers are increasingly favoring wearable biosensors with flexible properties that can be seamlessly integrated with human tissues, achieved through the utilization of soft materials. Gallium (Ga)-based liquid metals (LMs) possess desirable properties, such as fluidity, high conductivity, and negligible toxicity, which make them inherently soft and well-suited for the fabrication of flexible and wearable biosensors. In this article, we present a comprehensive overview of the recent advancements in the nascent realm of flexible and wearable biosensors employing LMs as key components. This paper provides a detailed exposition of the unique characteristics of Ga-based LM materials, which set them apart from traditional materials. Moreover, the state-of-the-art applications of Ga-based LMs in flexible and wearable biosensors that expounded from six aspects are reviewed, including wearable interconnects, pressure sensors, strain sensors, temperature sensors, and implantable bioelectrodes. Furthermore, perspectives on the key challenges and future developing directions of LM-enabled wearable and flexible biosensors are also discussed.
Keywords
INTRODUCTION
Wearable biosensors are directly worn inside or outside the body in the form of watches, bracelets, glasses, and clothing to acquire real-time and useful information related to heart rate[1-3], blood oxygen[4,5], exercise[6-8], and other physiological signals[9-11]. The key consideration for wearable biosensors is the comfort of individuals who wear the devices during routine activities without restricting sensing precision[12]. Conventionally, wearable biosensors have been fabricated from rigid materials, limiting their comfort during use and compromising safety for implantation. Thus, the development of biosensors with flexible and stretchable properties is crucial to allow for conformal integration onto human skin and tissue, facilitating optimal signal acquisition and transmission.
In recent years, gallium (Ga)-based liquid metals (LMs) have transcended their role in many fields, finding diverse applications across multiple disciplines. From soft robotics and advanced electronics to additive manufacturing and biomedicine, their exceptional properties have fostered innovation and paved the way for transformative breakthroughs in various fields[13-15]. Especially in the field of flexible and wearable electronics, attributed to their unique mechanical/chemical properties, Ga-based LMs have attracted much attention. These properties include (1) LMs are in a liquid state with good fluidity at room temperature, resulting in negligible Young’s modulus that can avoid the mechanical interface mismatch between the device and human tissue[15-17]; (2) they are metallic materials with high electrical/thermal conductivity, which are essential for conducting electricity and heat transfer of wearable biosensors[18,19]; and (3) the low toxicity of LMs satisfies the bio-friendly requirements of wearable electronics[20,21]. In addition, pattering technologies depending on the native physical properties of LMs, such as channel injection[22,23], direct writing[24-26], and spray-printing[27] methods, have greatly enabled the fabrication of LM-based wearable and flexible sensors. Various wearable biosensors that are mountable on human tissue have been developed using LMs. These biosensors have expanded the scope of wearable electronics and can be utilized in numerous areas, such as human-machine interface, motion detection, and health monitoring.
This review aims to summarize the basic physical properties of LM-based materials and provides an in-depth introduction to the designs/applications of flexible/stretchable interconnects, pressure sensors, strain sensors, and implantable electrodes based on LMs [Figure 1]. This review begins with providing a detailed description of the physical properties and electrically conductive mechanisms of LMs-based materials, including raw LMs, LM nanoparticles (LMNPs) inks, and LM-polymer composites. The second section summarizes recent advancements in LM-based biosensing devices, including interconnections, pressure sensors, strain sensors, and implantable bioelectrodes. These advancements include nano-scale LM circuits for improved bioelectronic integration, enhanced sensitivity of LM pressure sensors for monitoring gastrointestinal pressure, fiber-featured strain sensors for breathing monitoring, and implantable bioelectrodes capable of sensing electroencephalogram (EEG)/electrocardio (ECG) signals. These recently developed LM-based biosensors offer exciting opportunities for in vivo/vitro monitoring of heart rate, breath rate, body movement, and EEG/ECG signals. At last, the final section discusses the challenges and future research perspectives of LM-based biosensors.
GA-BASED LMs, LMNP INKs, AND LM-ELASTOMER COMPOSITES
Raw LMs
Ga and Ga-based alloys are the most popular used LMs for flexible electronics when compared to liquid-state metals, such as mercury (Hg), rubidium (Rb), cesium (Cs), and francium (Fr). This is due to their unique physical properties, such as relatively stable chemical properties in air and water, low melting point[15], high electrical/thermal conductivities[28,29], low viscosity[28,29], and low toxicity[30]. Pure Ga alloys with a melting point of 29.8 °C are solid-state at room temperature. The addition of In and Sn alloys through alloying can reduce the melting point (e.g., the melting point of Galinstan with 20.5 wt.% In and 12.5 wt.% Sn is 11 °C). One important characteristic of Ga-based LMs is their low crystallization temperature, which is significantly lower than their melting point. This phenomenon is known as the supercooling effect[31,32]. The supercooling effect enables Ga-based LMs to remain in a liquid state at temperatures far below their melting point, thereby expanding the working temperature range for LM-based flexible and wearable electronics. This feature is critical for ensuring the working stability of these devices. In addition, viscosity is one of the essential parameters for patterning bulk LM into specific 2D/3D structures. The viscosity of Ga-based LMs is comparably low, approximately twice that of water[33]. The viscosity of LMs can be adjusted through the alloying process. For instance, the viscosity of eutectic Ga-In (EGaIn) is 1.99 × 10-3 kg/m/s, which is lower than pure liquid Ga (2.04 × 10-3 kg/m/s).
Toxicity: Unlike mercury, Ga-based LMs can remain stable at room temperature, attributed to their negligible vapor pressure and solubility in water[19]. Although the toxicity of LMs remains controversial, more recent reports suggest that LMs are relatively safe for biological applications. Chitambar and White et al. have summarized the medical applications, toxicities, and health impacts of Ga and its compounds[34,35]. Ga-based LMs are relatively safe to be applied to medical treatment. For example, LM-printed electronics applied to the skin for tumor-treating field therapy exhibit no obvious side effects[36]. EGaIn/calcium alginate hydrogel can be used as a candidate for endovascular embolization and tumor embolotherapy with low toxicity[37]. Furthermore, Ga-based LM electrodes showed no adverse effects on the growth of neurons within the culture platform and were able to effectively simulate target neurons[38]. The mechanism of the low toxicity of LMs remains elusive, and it might relate to the following factors. On the one hand, Ga-based LMs can be degraded in body fluid, and the metabolites of LMs can be excreted to the outside of the body in the manner of both fecal and renal excretions[39]. On the other hand, for wearable flexible electronics, LMs are ordinarily encapsulated within biocompatible elastomers. Indirect contact between LMs and tissue further ensures the safety of biological applications. However, studies about the toxicity of LMs are unsystematic, and more rigorous research should be applied to understand the influence of LMs on human health. Nevertheless, it is essential to continue studying the toxicity of Ga-based LMs and to exercise caution during their fabrication and application processes[15,40].
Oxidation and Wettability: Similar to other metals such as aluminum, magnesium, and copper alloys, a self-limiting growth Ga2O3 oxide layer immediately forms on the surface of LMs when the oxygen concentration is higher than the ppm level[41]. The thickness of the oxide layer is associated with the oxygen concentration (~0.7 nm under vacuum conditions and much thicker at ambient conditions)[42-44]. It is elastic and stable under critical yield surface stress (~0.5-0.6 N/m). Only when the stress is above the yield strength, the oxide layer will rupture and the fresh LM flows out[44]. Attributed to the oxide layer, the wettability and surface tension of LM droplets are significantly altered. Furthermore, oxidizing LMs is an important method to control the wettability for patterning circuits on different substrates[45-47].
LMNP ink
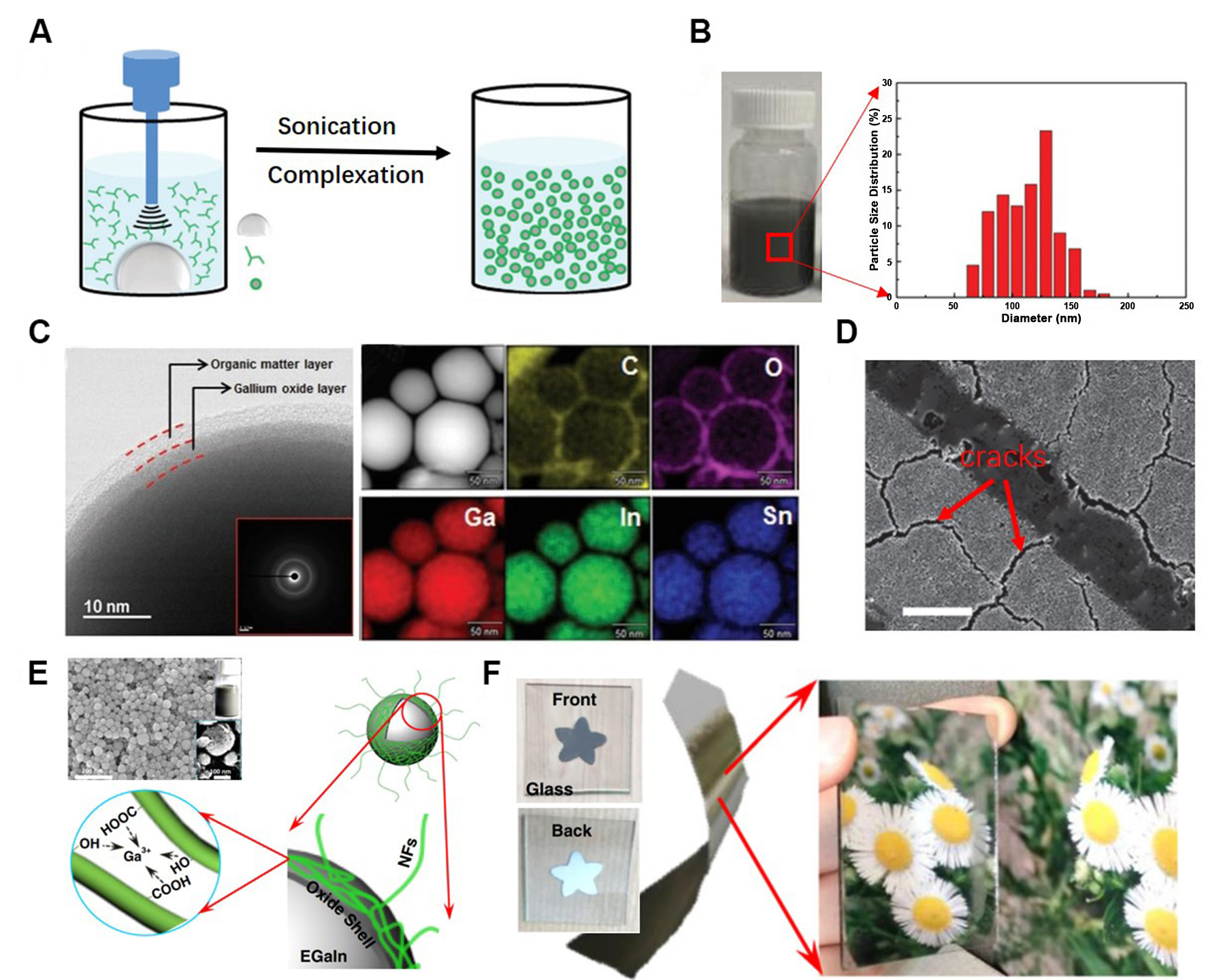
Although Ga-based LMs possess tremendous advantages for the fabrication of flexible and wearable biosensors, the ability to fabricate biosensors using high-efficiency preparation methods remains constrained by their high surface tension[44,47]. Besides, directly using raw LMs as the patterning material leads to high resource consumption, which will increase the costs and limit their market competitiveness[48]. To solve this problem, LMNP inks were developed as the pattering materials through sonicating bulk LMs in organic solvents, as shown in Figure 2A and B. Acoustic cavitation generated by the probe induces the transformation of bulk LMs into LMNPs, while polymers containing anchoring groups, such as thiols, trithiocarbonate, phosphate, and silane, serve to colloidally stabilize the LMNPs[20,37,49-53]. The particle size distribution of LMNP inks is associated with output power, sonication time, and solution temperature[50]. Figure 2C shows electron microscopy images and element mappings of LMNPs, demonstrating the core-shell structure with ~3 nm thick organic matter layers and amorphous Ga oxide layers. However, the existence of an oxide layer and cracks induced by the internal stress produced by unsymmetrical capillary forces make the circuit prepared by LMNP inks nonconductive [Figure 2D][49]. In order to form a conductive pathway, sintering processes, such as mechanical sintering[49,54], laser sintering[55,56], and evaporation sintering[37], should be used to break oxide shells of LMNPs for connecting fresh LM core. Li
Figure 2. The fabrication method and microstructure characterization of LMNP inks. (A) Synthesize LMNPs by a probe sonication method. Reproduced with permission[50]. Copyright 2016, John Wiley and Sons; (B) Photo and particle size distribution of LMNP inks. Reproduced with permission[50]. Copyright 2016, John Wiley and Sons; (C) High Resolution Transmission Electron Microscope (HRTEM) and scanning transmission electron microscopy (STEM) images, along with elements mapping of LMNPs. Reproduced with permission[50]. Copyright 2016, John Wiley and Sons; (D) Scanning electron microscope (SEM) image of the circuit patterned using LMNP inks[49]. Scale bar is 20 m. Reproduced with permission. Copyright 2015, John Wiley and Sons; (E) Schematic illustration of EGaIn droplets encapsulated in oxide shell and with CNFs attached on the surface via interactions with Ga3+. Reproduced with permission[57]. Copyright 2019, The Authors; (F) Evaporation-induced sintering films with mirror-like bottom surface and grey top surface. Reproduced with permission[57]. Copyright 2019, The Authors. CNFs: Cellulose biological nanofibrils; EGaIn: eutectic Ga-In; Ga: gallium; LM: liquid metal; LMNP: LM nanoparticle.
LM-elastomer composites
LM particles can be used as an ideal alternative to rigid fillers for making conductive composites. LM-elastomer composites combine the electrical, mechanical, and thermal properties of the filler and the elastomeric matrix[58-60]. The composite is usually prepared by shear mixing the precursor elastomer and LM, and the composite properties, such as conductivity and elastic modulus, can be regulated by the mixing time, mixing revolutions per minute (RPM), and curing temperature [Figure 3A and B][61,62]. Compared to rigid fillers, LM fillers offer a composite with some unique electrical properties. For example, LM composites with the autonomously self-healing properties have been demonstrated [Figure 3C][60]. When the composite is mechanically damaged, the droplets rupture to form new connections with neighboring droplets and restore the electrical function without human intervention or the application of external heat. Temperature-controlled reversible electrical transition between insulator and conductor properties has been achieved using LM fillers
Figure 3. The preparation process and microstructure characterization of LM-elastomer composites. (A) The overview of processes for preparing LM inclusion composites. Reproduced with permission[61]. Copyright 2020, John Wiley and Sons; (B) Photograph of a kind of LM-elastomer composite for printing circuits. Reproduced with permission[62]. Copyright 2019, American Chemical Society; (C) An LM composite with self-healing performances being stretched and twisted. Reproduced with permission[60]. Copyright 2018, The Authors; (D) A schematic illustration of mechanisms in electrical transition between insulative and conductive LM composites to temperature change. Reproduced with permission[63]. Copyright 2019, John Wiley and Sons; (E) Microstructure characterization of LM-elastomer composites with anisotropic and unconventional piezoconductivity. Reproduced with permission[64]. Copyright 2020, Elsevier. EGaIn: Eutectic Ga-In; LM: liquid metal; PDMS: polydimethylsiloxane; RPM: revolutions per minute.
WEARABLE BIOSENSORS
Wearable interconnects
Interconnects are the most basic components for connecting electronic elements, such as sensors, resistors, and capacitors. High electrical conductivity, ease of patterning, high stretchability, and non-toxicity properties make LMs one of the best candidates for the fabrication of wearable circuits. Ga-based LMs in the forms of raw materials[71,72], LM composites[70,73], and LMNP inks[20,74] have been demonstrated to connect rigid electrical components. They can significantly improve the flexibility and electrical performance of wearable devices for healthcare applications, such as electrical stimulation, electrochemical sensing, and temperature monitoring.
Among raw LMs, LMNP inks, and LM composite materials, raw LMs are the most widely studied and used materials for the preparation of interconnects. For 3D-structured interconnects, LM fibers prepared by directly injecting raw LMs into microchannels have been applied for digital-embroidered electronic textiles[72], magnetic resonance imaging (MRI) detectors[75], and biomimetic eyes[76] [Figure 4A-C]. LM fibers are highly flexible and conductive, allowing them to be used in 3D spaces without any spatial constraints. This greatly expands the potential applications of LM circuits due to their adaptability to different environments. For 2D circuits, with the development of advanced patterning technologies[23], the resolution of LM circuits has been significantly improved, which is of great significance in improving the integration of wearable devices. As shown in Figure 4D-F, LM interconnects with the widths of 250 μm, 30 μm, and even 500 nm can be prepared by injection[77-79], transfer printing[80,81], and lithography methods[82,83], respectively. Interconnects prepared by raw LMs have metal-like electrical conductivity and outstanding stretchability, and the stretchability is only limited by the elastomer that encapsulates LMs. On the other hand, the use of bulk LMs may present challenges as it is susceptible to leakage. In addition, establishing a reliable connection with electronic components can be challenging as the LM must be able to effectively wet the surface of the component[84]. Therefore, LMNP inks and LM composites have been developed for the fabrication of interconnects, which can significantly enhance the wettability to form good connections. Compared to raw LM materials, LMNP inks have the advantages of compatibility with diverse substrates and ease of scalability and allow for automatic fabrication methods. For example, Liu et al. proposed a spray-printing strategy for the scalable fabrication of soft and flexible circuits based on a LMNP ink, but an additional sintering process is needed to rupture and ablate the LMNP Ga2O3 shell to allow the LM cores to escape and coalesce [Figure 4G][55].
Figure 4. The circuits prepared using LM-based materials. (A) Digital-embroidered electronic textiles prepared by LM fibers. Reproduced with permission[72]. Copyright 2020, The Authors; (B) LM conductors for MRI detectors. Reproduced with permission[75]. The Authors; (C) Scheme of a biomimetic eye using LM fibers as the circuits. Reproduced with permission[76]. Copyright 2020, Springer Nature; LM circuits with the width of (D) 250 μm. Reproduced with permission[78]. Copyright 2019, John Wiley and Sons; (E) 30 μm. Reproduced with permission[81]. Copyright 2020, John Wiley and Sons and (F) 500 nm. Reproduced with permission[82]. Copyright 2020, The Authors; (G) Circuits prepared using LMNP inks and sintered by laser. Reproduced with permission[55]. Copyright 2018, American Chemical Society; (H) The circuits prepared using LM composite before and after the activation. Reproduced with permission[85]. Copyright 2021, American Chemical Society; (I) LM composite circuits for “Island-bridge” structure of an energy harvesting system[86], scale bar is 5 mm. Reproduced with permission. Copyright 2020, John Wiley and Sons. LM: Liquid metal; LMNP: LM nanoparticle; MRI: magnetic resonance imaging.
LM-elastomer composites can further be used to enhance the working reliability of LM interconnects
Wearable sensors
Wearable sensors affixed to garments or directly onto the human skin have emerged as crucial tools for real-time activity monitoring. Wearable devices necessitate highly reliable sensors that can conform to the curvilinear surface of the human body with minimal discomfort. LM-based materials are outstanding candidates for preparing wearable sensors due to their fluidic nature and biocompatibility. Due to the fluidity of LMs and low elastic modulus of encapsulating polymers, LM channels inside the polymer can be deformed upon the application of external forces, inducing the change of electrical signals. LM-based flexible and wearable sensors have recently demonstrated immense potential for various applications, including healthcare monitoring[23,92], disease early warning[93], motion detection[26,62,94,95], and soft robotics[96]. These sensors, particularly stretchable and skin-mountable variants, can function as pressure, strain, optical, and temperature sensors, as elaborated in detail below.
Pressure Sensors: Wearable LM-based pressure sensors commonly use resistance or capacitance-based detection methods to sense pressure changes. Most LM pressure sensors detect the change of resistance. The LM encapsulated within a fluid channel deforms when an external force is applied to it
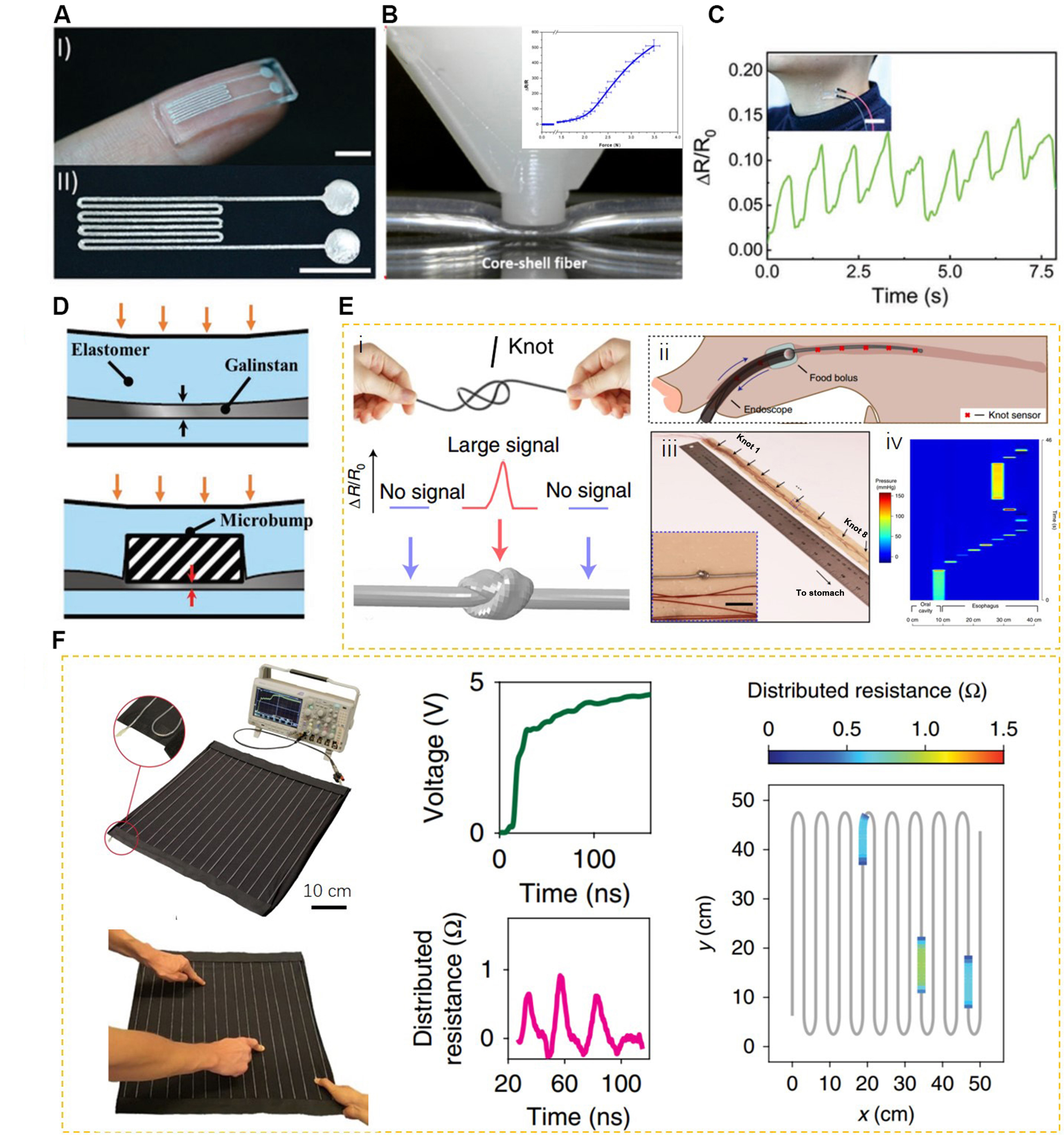
Figure 5. Pressure sensors prepared using LMs; (A) Photograph of the pressure sensor prepared by a channel filling method, scale bars are 5 mm. Reproduced with permission[97]. Copyright 2019, John Wiley and Sons; (B) Photograph for testing the performance of a fiber-structured pressure sensor, and the insert is the resistance change R/R0 under different force loadings. Reproduced with permission[79]. Copyright 2020, Elsevier B.V; (C) The resistance change of LM pressure sensors prepared by channel filling method for monitoring human neck pulses. Reproduced with permission[97]. Copyright 2019, John Wiley and Sons; (D) Schematic illustration of the highly sensitive pressure sensor enhanced by a rigid micropump. Reproduced with permission[103]. Copyright 2019, John Wiley and Sons; (E) Enhanced sensitivity of LM pressure sensors by resembling the quipu-knotted strings used by Andean civilizations for monitoring the gastrointestinal pressure. Reproduced with permission[109]. Copyright 2022, Springer Nature; (F) Soft and stretchable LM transmission lines as distributed probes of multimodal deformations. Reproduced with permission[113]. Copyright 2020, Springer Nature. LM: Liquid metal.
The sensitivity of a sensor is a critical parameter for sensing applications, and research has demonstrated that the Poisson’s ratio of the material, the thickness of the top layer, and the dimension of microchannels all play significant roles in the sensitivity of microchannel-based pressure sensors[106,107]. The pressure sensitivity of previously reported LM-based pressure sensors is within the range of 0.2-80 × 10-3 kPa-1, and such sensitivity is not optimal due to the relatively thick elastomer dielectric layer[103]. Therefore, strategies, such as microchannel cross-sectional geometry design for enhancing the penetrating effect of channel base[108], ‘S’-shaped microfluidic structure design with a higher and sharper deformation profile[102], and Wheatstone bridge circuit design[23], have been proposed to improve the sensitivity. Kim et al. introduced 3D-printed rigid microbumps on the top of an LM microchannel that can offer extremely high sensitivity (0.158 kPa-1) compared with traditional straight channel LM pressure sensors (0.2-80 × 10-3 kPa-1), as shown in Figure 5D[103]. Furthermore, a low-cost and highly-sensitive LM pressure sensor for gastrointestinal manometry was prepared to refer to the quipu-knotted strings, as shown in Figure 5E[109]. By simply typing knots on the EGaIn-filled fiber, a small pressure of less than 50 kPa can be detected, while no signal can be detected when the same pressure is applied to the unknotted region. The enhanced sensitivity is attributed to the amplification of the effective total pressure induced by the folded and stacked channel layers. For application demonstration, the designed ribbon-like manometry device with eight knots can clearly record the backflow and retention of artificial food bolus by evaluating the oesophageal pressure [Figure 5E ii and iii].
Quantifying and spatially sensing dynamic multipoint deformations remain a challenge. Skin-like multi-tactile sensors have shown significance for acquiring tactile information of multiple points[110-112]. For LM-based multi-tactile sensors, a two-layer core-shell fiber-based tactile sensor has been designed and fabricated via the coaxial ink writing of a continuous single core-shell fiber[79]. When two fingers are placed on the two sensing nodes, the resistances of both the top and bottom layer fibers of the sensing nodes are simultaneously recorded. The resistance values can be used to determine the specific position of the fingers. Furthermore, based on time-domain reflectometry, Leber et al. proposed a soft and stretchable LM transmission line as distributed probes for sensing multimodal deformations, as shown in Figure 5F[113]. In this sensor, soft transmission line probes were integrated into a piece of stretchable fabric, and a custom pulse generator was used in conjunction with a standard laboratory oscilloscope to induce time-domain reflection. When the transmission line is pressed, steps of signal appear in the waveform and peaks equal to the number of pressed points emerge in the distributed resistance profile, the height of which corresponds to the applied pressure. These points can then be accurately positioned on the spatial map.
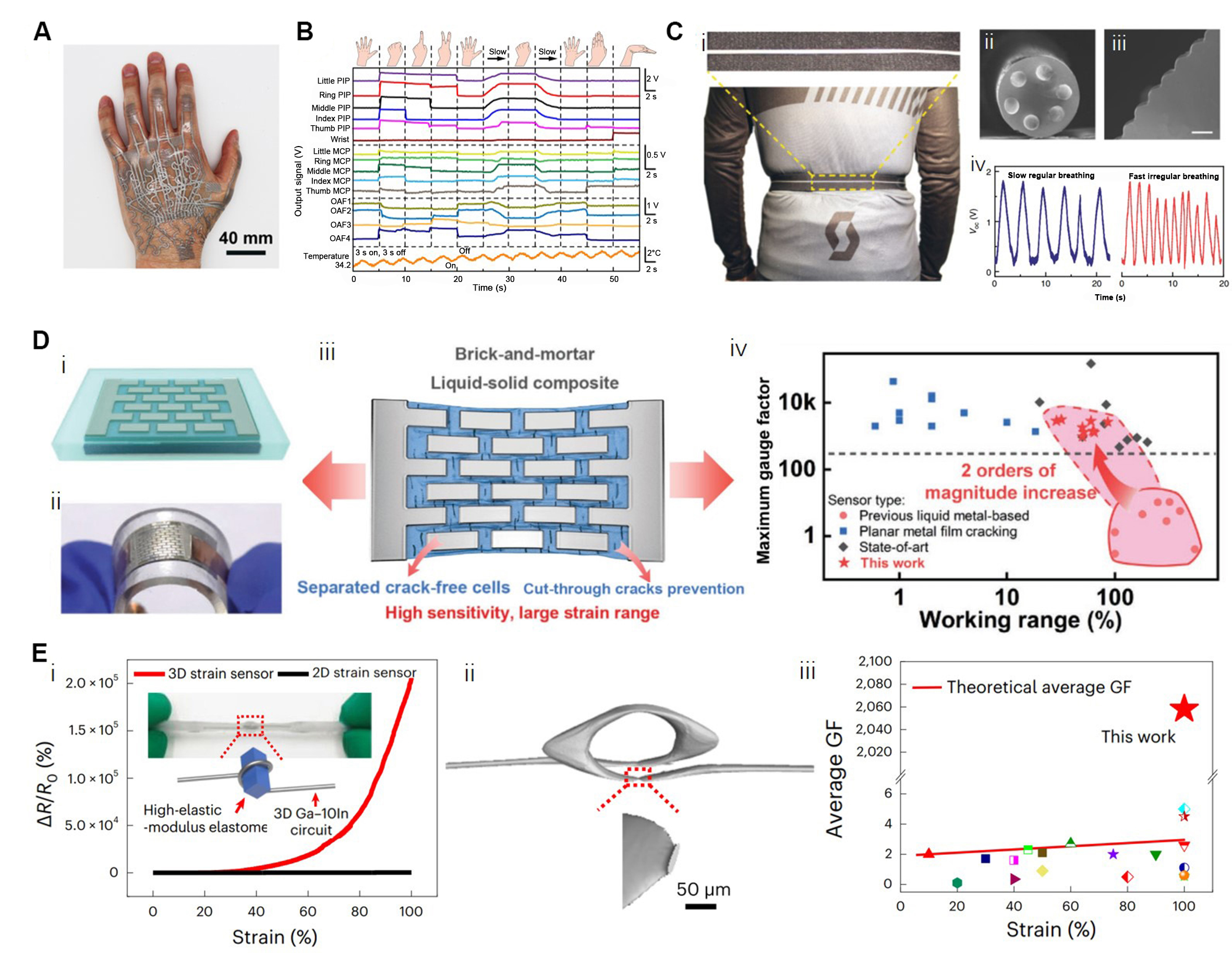
Strain Sensors: Similar to LM pressure sensors, sensors based on the change of resistance[114,115], capacitance[116], and resonant frequency[117] have been employed to detect strain. For LM-filled fiber and microchannel strain sensors, LM elongates along the strain direction, leading to an increase of resistance that conforms to the equation R = R0 (1 + ε)2 (R0: initial resistance, ε: strain, R: the resistance corresponds to ε). For most applications, these types of strain sensors are integrated into wired/wireless gloves to detect hand gestures by monitoring the output voltage or resistance in real-time[25,26,48,87,114]. Tang et al. developed a layer-by-layer fabrication method for integrating strain sensors with a multilayer electronic transfer tattoo that can be stretched up to 800% strain and conformably attached to the skin [Figure 6A][118]. This tattoo can amplify the output signal of integrated strain sensors by three times and achieve the monitoring of hand movements in real time [Figure 6B]. In addition, Dong et al. proposed a fiber strain sensor with a surface texture and six LM electrodes to realize the breath monitoring based on self-powered sensing
Figure 6. LM-based strain sensors. (A and B) An LM-based multilayer tattoo integrated with stretchable strain sensors can preciously monitor the hand movements in real time. Reproduced with permission[118]. Copyright 2021, American Association for the Advancement of Science; (C) Breathing monitoring by a self-powered triboelectric fiber featured strain sensor with six embedded electrodes and surface texture. Reproduced with permission[92]. Copyright 2020, Springer Nature; (D) LM-based and nacre-inspired strain sensors, the brick-and-mortar architecture can control the micrograph of cracks; thus, the sensitivity was increased by about two orders of magnitude. Reproduced with permission[94]. Copyright 2021, John Wiley and Sons; (E) Resistance response, microstructure characterization, and sensitivity comparison of a high-sensitive 3D-structured LM strain sensor. Reproduced with permission[120]. Copyright 2023, All authors. Ga: Gallium; GF: gauge factor; LM: liquid metal.
The gauge factor (GF), which reflects the sensitivity of the LM-based straight channel strain sensor, is relatively low (below 5 under 100% strain). Theoretically, the relatively low average GF of a straight-channel strain sensor, as described by the formula GF = ε + 2, fails to meet the criteria set for commercial wearable strain sensors. To increase the sensitivity, Kramer et al. introduced a curvature sensor with a hollow structure, in which an embedded strut can exert pressure on the microchannel during bending, leading to a greater change in resistance[119]. Moreover, a nacre-inspired and LM-based ultrasensitive strain sensor by a spatially regulated cracking strategy has been developed [Figure 6D i and ii][94]. The biphasic pattern (LM with Cr/Cu underlayer) acts as “bricks”, and strain-sensitive Ag film acts as “mortar”. Compared to LM conductors, this strategy allows the conductive pathways to form a certain number of cracks under strain
Temperature sensors: Temperature is a critical and fundamental parameter to evaluate human health. The volume expansion of fluid in a closed pipe correlated to a specific temperature value is a commonly used principle in thermometers, as seen in the traditional mercury thermometer. In contrast to mercury-based thermometers, the preeminent merit of Ga-based thermometers for wearable biosensors is their neglected toxicity. This remarkable trait, coupled with their supercooling performance, empowers Ga-based thermometers-founded upon the principle of volume expansion-to reliably operate in environments as frigid as -5 °C and, in some cases, even down to -10 °C. Evidencing their practicality, medical-grade Ga-based thermometers from esteemed manufacturers, such as Geratherm and Mediblink, have gained substantial traction in commercial utilization.
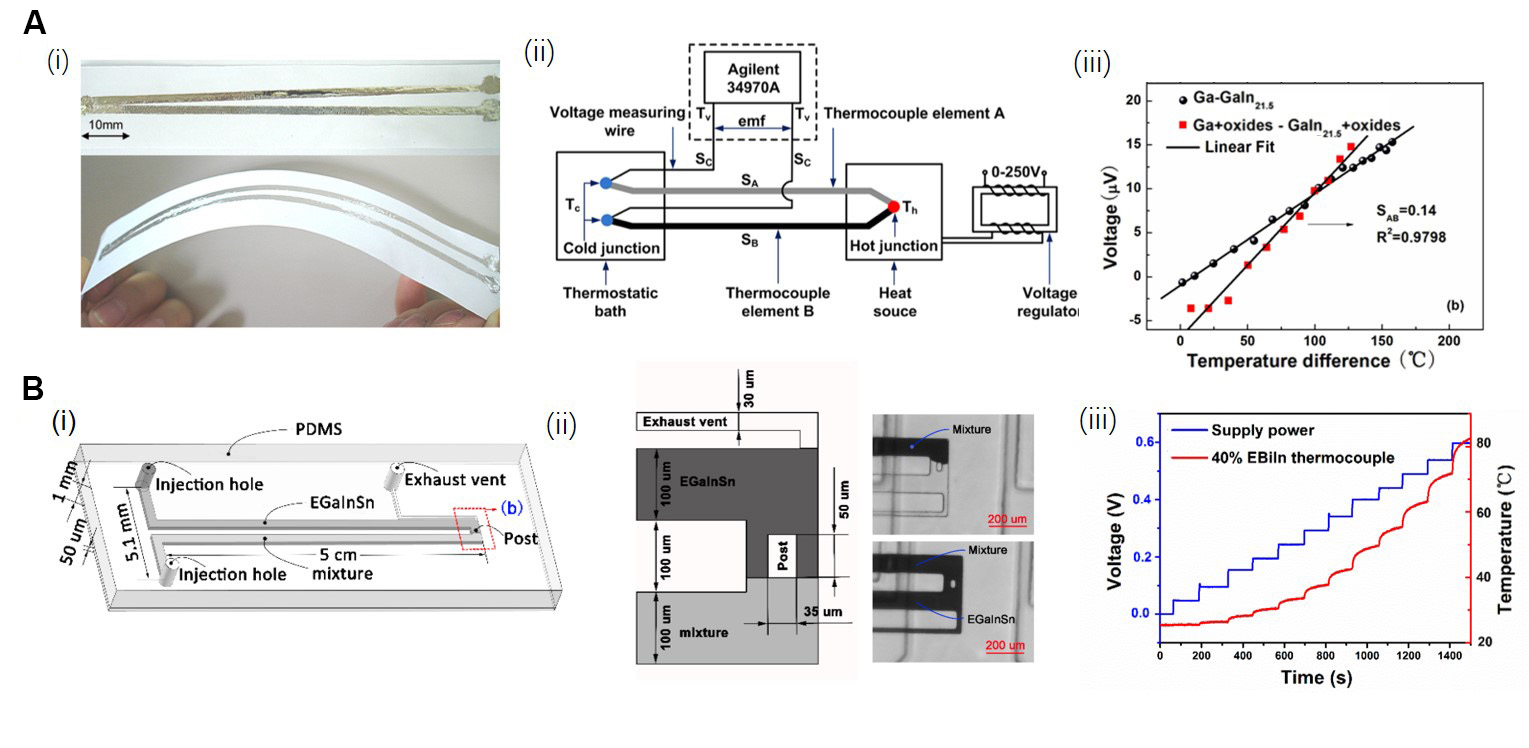
The Seebeck effect is another commonly used principle for designing and fabricating thermometers in the industry[121,122] and occurs when the ends of a thermocouple are subjected to a temperature difference, resulting in an electrical current flowing between them. Thermometers prepared based on the Seebeck effect exhibit much higher sensitivity compared to those utilizing the volume expansion principle[107]. Li et al. prepared a thermocouple using Ga and EGaIn as the materials of two branches, respectively
Figure 7. Temperature sensors designed and prepared using the LMs. (A i) Photograph of the printed thermocouple prepared using Ga with 0.25 wt.% oxides- GaIn21.5 with 0.25 wt.% Ga oxides, and the thin film thickness is about 50 μm; (A ii) Schematic diagram of a printable tiny thermocouple prepared by LMs; (A iii) The measured thermoelectric voltage as a function of temperature difference using the thermocouple in (A i). Reproduced with permission[123]. Copyright 2012, AIP publishing; (B i) Schematic of a handy micro-thermocouple, two poles of the channel are filled with EGaInSn and Bi-based metal-alloy mixture, respectively; (B ii) The EGaInSn and mixture converged at the middle of the channel; (B iii) Performance of the thermocouple in (B i). Reproduced with permission[124]. Copyright 2019, Multidisciplinary Digital Publishing Institute. EGaIn: Eutectic Ga-In; Ga: gallium; PDMS: polydimethylsiloxane.
Implantable bioelectrodes
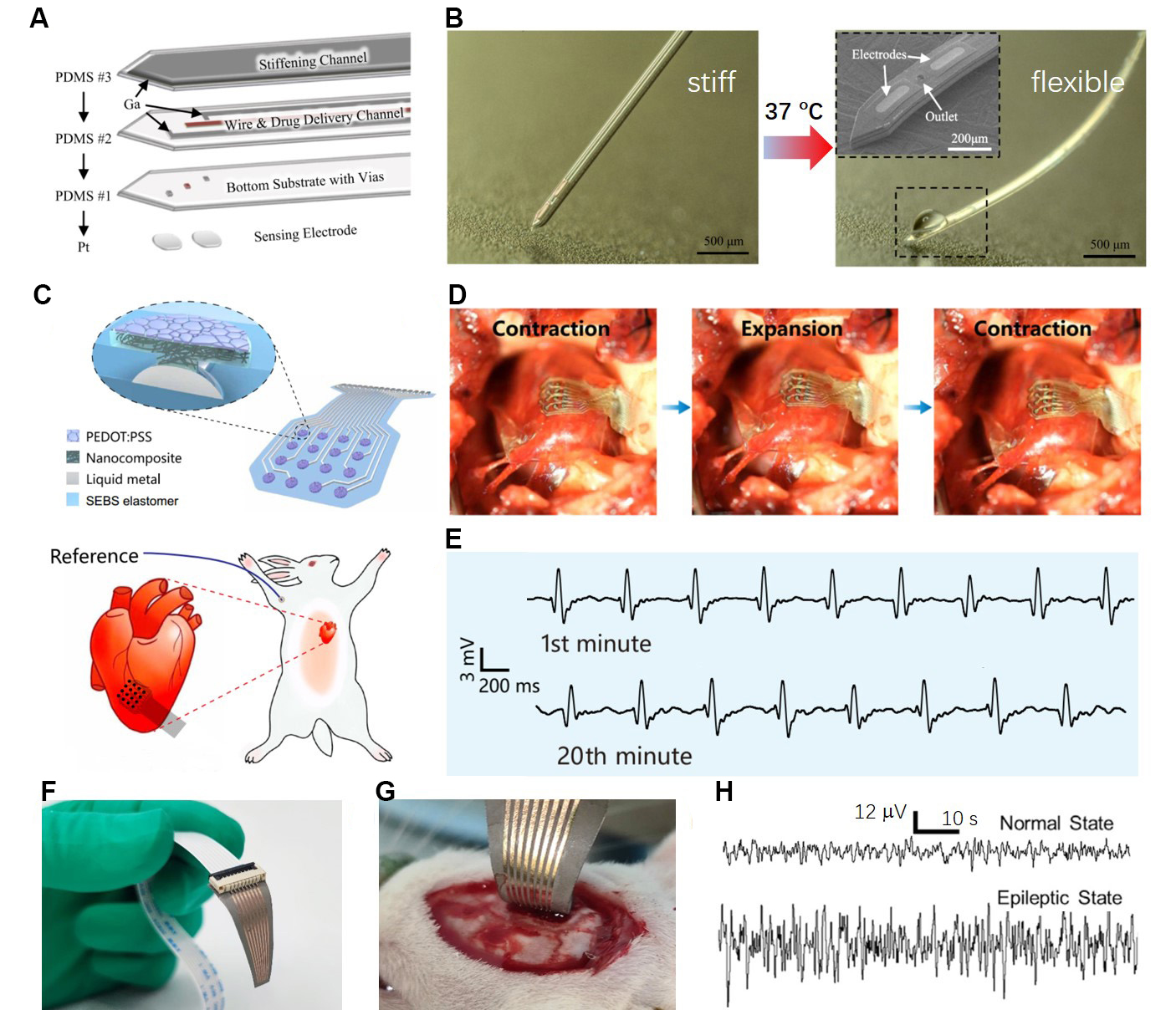
Ga-based LMs have shown great potential for bio-related applications due to their excellent electrical conductivity, fluidic properties, and negligible toxicity. Reports suggest that LMs can be directly patterned onto the skin for ECG monitoring[125-127], fabricated into external stents and electronic blood vessels[128,129], and acted as nerve connectors[130]. Despite the promising properties of LM-based stretchable and implantable electronics, there are still practical issues that need to be addressed. One major concern is the fluidic nature of LMs, which can lead to insufficient structural stability when in direct contact with dynamically moving organs and tissues. This lack of stability can compromise the security and reliability of the electronics. One approach to solve this problem is to alter the rheology of LMs by increasing the content of In in a Ga-In alloy. For instance, Timosina et al. reported that the Ga-In alloy with 50 wt% of In has a non-Newtonian shear-thinning property, exhibiting high viscosity when still, and can flow similarly to an LM when sheared[127]. This property can prevent material leakage and enhance processability. Also, the rapid formation of an insulating Ga2O3 layer severely affects the conductive performance between LMs and tissues. Another challenge with using LMs for long-term service in bioelectronics applications is their susceptibility to chemical corrosion under physiological conditions. This presents significant challenges to the efficiency and safety of the devices. To address this issue, LMs are often encapsulated in bio-friendly polymers for flexible bioelectronics applications. By using LMNPs, Dong et al. proposed an LM electrode array prepared using the screen-printing method for in vivo neural recording[131]. In addition, by utilizing the low melting point property of pure Ga, a flexible and multifunctional neural probe with ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery has been developed, as shown in Figure 8A[132]. This neural probe was designed with three layers, and pure Ga LMs were used to fill the top stiffening channel layer and medium conductive channel. At room temperature, the neural probe is stiff due to the solid state of pure Ga. When the probe is inserted into the brain, the body temperature will induce the melting of Ga so that the neural electrodes restore flexibility [Figure 8B].
Figure 8. Implantable flexible electrodes for sensing biosignals. (A) The exploded-view drawing of the ultra-large tunable stiffness electrodes enabled by LMs. Reproduced with permission[132]. Copyright 2019, Elsevier B.V; (B) The stiff sate electrode can translate into soft sate due to the melting of LMs, and the insert picture shows the outlet of the drug delivery channel and Pt electrodes of the probe tip. Reproduced with permission[132]. Copyright 2019, Elsevier B.V; (C) Schematic illustration of the highly stretchable electrodes for in vivo epicardial recording on a rabbit. Reproduced with permission[134]. Copyright 2022, American Association for the Advancement of Science; (D) The electrodes can be conformally attached to the right ventricle for long-term monitoring of the electrocardio; and (E) shows the representative electrogram for 20 min of monitoring. Reproduced with permission[134]. Copyright 2022, American Association for the Advancement of Science; (F) Photo of the stretched neural electrode arrays prepared by depositing Au film on LM-PDMS composite. Reproduced with permission[135]. Copyright 2022, All authors; (G) Intraoperative image of the neural electrode arrays for in vivo recording of ECoG signals, showing the high flexibility of the electrode to be in contact with the cerebral cortex of the rat; and (H) ECoG signals of a healthy rat under normal state and epileptic state. Reproduced with permission[135]. Copyright 2022, All authors. ECoG: Electrocorticogram; LM: liquid metal; PDMS: polydimethylsiloxane; PEDOT:PSS: poly 3,4-ethylene dioxythiophene : polystyrene sulfonate; SEBS: styrene-ethylene-butylene-styrene.
Although electrode arrays show stable electrical properties under high strain, the deposited solid-state conductors at the sensing site significantly affect the long-term stability due to the mechanical mismatch of the interface between the metal and polymer. Therefore, deposited conductive polymers have been adapted to encapsulate LM interconnects inside the polymer[133,134]. Wang et al. selected carbon nanotube composites and microcracked conductive poly 3,4-ethylene dioxythiophene : polystyrene sulfonate (PEDOT:PSS) polymer as the sensing sites of electrodes, as shown in Figure 8C[134]. Combining LM interconnects with a highly stretchable styrene-ethylene-butylene-styrene (SEBS) elastomer allows the electrode array to exhibit ultrahigh stretchability (up to 400% tensile strain) and a low interfacial impedance. In addition, the long-term in vivo epicardial recording on a rabbit [Figure 8D and E] further demonstrates the vast potential of the electrodes for health monitoring applications. Li et al. fabricated a stretchable 8-channel neural electrode array by depositing an Au film onto the surface of an LM-PDMS composite [Figure 8F][135]. The electrode array can conformally match the cortical surface of a rat due to its high flexibility and stretchability [Figure 8G]. The electrode array was effectively utilized for the in vivo identification of epilepsy by monitoring electrocorticogram (ECoG) signals [Figure 8H]. However, there is still a risk of leakage of raw LMs in implantable bioelectrodes. Therefore, we propose that implantable bioelectrodes based on LM-elastomer composites should be given more attention in future research, as they have the potential to address the issue of LM leakage.
CONCLUSIONS AND OUTLOOKS
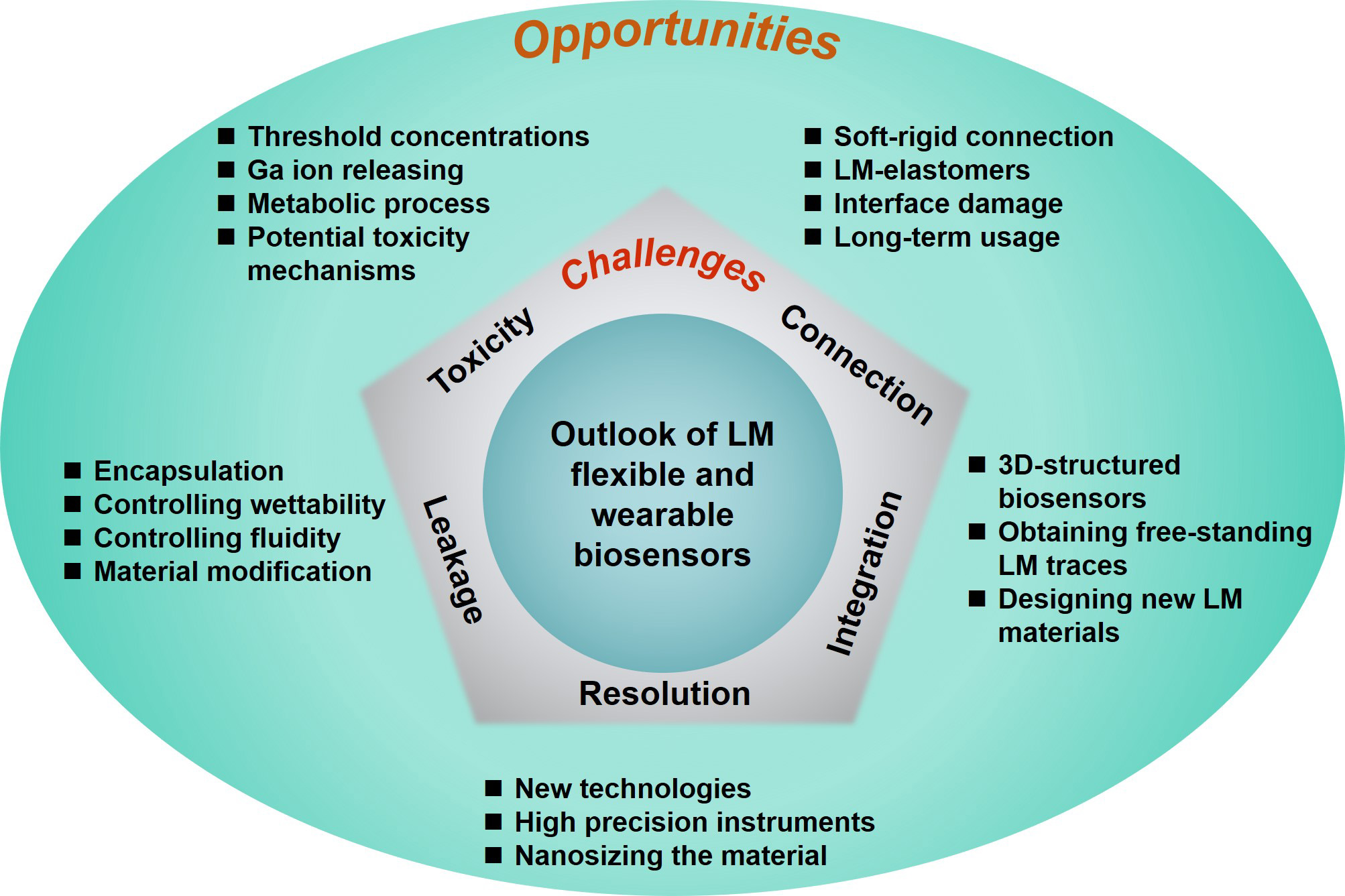
Unique properties such as fluidity, metallic electrical/thermal conductivity, and low toxicity of LMs contribute to their broadening applications of biosensors in the healthcare area. This review illustrated the recent advances in flexible and wearable LM-based biosensors, including interconnects, pressure sensors, strain sensors, temperature sensors, and implantable electrodes. However, there remain some challenges and opportunities associated with Ga-based biosensors, which are shown in Figure 9 and summarized below:
Figure 9. Prospects for challenges and opportunities in Ga-based wearable biosensors. Ga: Gallium; LM: liquid metal.
(1) Despite that many works have reported the low cytotoxicity of Ga and its alloys, it deserves to be noted that some reports revealed that Ga-based LMs are toxic at the cellular level[136,137], and the threshold concentrations of Ga, Ga-based alloys, and Ga ions need to be systematically investigated by conducting in vitro/vivo experiments. An important consideration when using bulk Ga-based LMs in biomedical applications is whether the Ga ions released from these materials can be absorbed through the skin[127]. In addition, further research is needed to investigate the metabolic process and potential toxicity mechanisms of Ga ions in the body[138].For instance, further investigations are warranted to elucidate the mechanism by which Ga ions induce damage to normal cellular components.
(2) Advanced connection technologies should be further developed to connect flexible LM biosensors to rigid electrical components. Currently, most LM-based biosensors use rigid metal wires to connect the encapsulated LM patterns with the external printed circuit board. However, the soft-rigid connection between the LM patterns and the wires is a weak point due to the mechanical mismatch between the two materials. For example, the rigid metal wire is very easy to be pulled out from the polymer. Besides, LMs would leak along the interface between the rigid metal wire and polymer while the biosensor is under long-term usage. Therefore, it is urgent to develop a more reliable encapsulation method to connect LM patterns and rigid electrical components. We recommend that the conductive LM-elastomers possess the potential to address the connection problem.
(3) The fluidic nature of LMs makes them susceptible to leakage from the encapsulating polymer, which can lead to contamination. This risk is exacerbated by stress concentrations generated by external forces during long-term stretching or folding. The leaked Ga-based LM can solder with electrical components without heating, which is destructive for electronic circuits. To prevent leakage of LM during long-term usage, effective encapsulation measures should be taken. Additionally, controlling the fluidity and wettability of LMs through oxidation, mixing with solid metal microparticles, and the development of novel LM-elastomer composites are effective strategies to address this problem.
(4) The resolution of LM-based biosensor patterns is another area that requires attention. Although LM nano-patterns with ~500 nm line width have been fabricated using lithography, the resolution is relatively lower than that achievable on a silicon wafer. High resolution is crucial for the integration of LM-based biosensors into flexible electronics. Therefore, future research should focus on developing precision instruments and optimizing processes, such as oxide formation and phase transition, during the patterning process to improve the resolution of LM patterns.
(5) One last issue that needs to be considered is the integration of LM-based biosensors. In addition to increasing the LM patterns resolution, fabricating 3D-structured biosensors is another effective solution to improve the integration of biosensors. However, obtaining free-standing LM traces and stable interconnects is challenging due to the fluidity of LMs. The commonly used microchannel filling methods and 3D printing methods cannot meet the requirements of efficient fabrication of highly integrated biosensors. We believe it is necessary to design new LM materials, such as alloys with plastic deformation performance, to develop a highly efficient fabrication method for highly integrated LM biosensors. In addition, creating multifunctional 3D printing holds a prominent advantage for achieving high precision and integrated 3D-structured biosensors.
DECLARATIONS
Authors’ contributions
Initiated the reviewing idea and outlined the manuscript structure: Xu Z, Ma X, Guo J
Involved in the discussion and revised the manuscript: Tang SY
Conducted the literature review and wrote the manuscript draft: Li G, Liu S
All authors have read the manuscript and approved the final version.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/V008382/1), the National Natural Science Foundation of China (92163109), Shenzhen Science and Technology Program (KQTD20170809110344233), and the Fundamental Research Funds for the Central Universities (Grant No. HIT.OCEF.2021032).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023.
REFERENCES
1. Ma B, Xu C, Chi J, Chen J, Zhao C, Liu H. A versatile approach for direct patterning of liquid metal using magnetic field. Adv Funct Mater 2019;29:1901370.
2. Zhou X, Zhang Y, Yang J, Li J, Luo S, Wei D. Flexible and highly sensitive pressure sensors based on microstructured carbon nanowalls electrodes. Nanomaterials 2019;9:496.
3. Jiang Y, Liu Z, Matsuhisa N, et al. Auxetic mechanical metamaterials to enhance sensitivity of stretchable strain sensors. Adv Mater 2018;30:e1706589.
4. Li H, Xu Y, Li X, et al. Epidermal inorganic optoelectronics for blood oxygen measurement. Adv Healthc Mater 2017;6:1601013.
5. Jiao B. Anti-motion interference wearable device for monitoring blood oxygen saturation based on sliding window algorithm. IEEE Access 2020;8:124675-87.
6. Liu Z, Qi D, Hu G, et al. Surface strain redistribution on structured microfibers to enhance sensitivity of fiber-shaped stretchable strain sensors. Adv Mater 2018;30:1704229.
7. Liao X, Zhang Z, Kang Z, Gao F, Liao Q, Zhang Y. Ultrasensitive and stretchable resistive strain sensors designed for wearable electronics. Mater Horiz 2017;4:502-10.
8. Zhang M, Wang C, Wang H, Jian M, Hao X, Zhang Y. Carbonized cotton fabric for high-performance wearable strain sensors. Adv Funct Mater 2017;27:1604795.
9. Maier D, Laubender E, Basavanna A, et al. Toward continuous monitoring of breath biochemistry: a paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath. ACS Sens 2019;4:2945-51.
10. Veeralingam S, Khandelwal S, Sha R, Badhulika S. Direct growth of FeS2 on paper: a flexible, multifunctional platform for ultra-low cost, low power memristor and wearable non-contact breath sensor for activity detection. Mat Sci Semicon Proc 2020;108:104910.
11. Liu Z, Wang H, Huang P, et al. Highly stable and stretchable conductive films through thermal-radiation-assisted metal encapsulation. Adv Mater 2019;31:1901360.
12. Jeong YR, Lee G, Park H, Ha JS. Stretchable, skin-attachable electronics with integrated energy storage devices for biosignal monitoring. Acc Chem Res 2019;52:91-9.
13. Wei C, Tan L, Tao Y, et al. Interfacial passivation by room-temperature liquid metal enabling stable 5 V-class lithium-metal batteries in commercial carbonate-based electrolyte. Energy Stor Mater 2021;34:12-21.
14. Wei C, Tan L, Zhang Y, et al. Highly reversible Mg metal anodes enabled by interfacial liquid metal engineering for high-energy Mg-S batteries. Energy Stor Mater 2022;48:447-57.
16. Zhu S, So J, Mays R, et al. Ultrastretchable fibers with metallic conductivity using a liquid metal alloy core. Adv Funct Mater 2013;23:2308-14.
17. Dickey MD, Chiechi RC, Larsen RJ, Weiss EA, Weitz DA, Whitesides GM. Eutectic Gallium-Indium (EGaIn): a liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv Funct Mater 2008;18:1097-104.
18. Yang X, Tan S, Liu J. Numerical investigation of the phase change process of low melting point metal. Int J Heat Mass Transf 2016;100:899-907.
19. Liu S, Sweatman K, McDonald S, Nogita K. Ga-based alloys in microelectronic interconnects: a review. Materials 2018;11:1384.
20. Zhang M, Li G, Huang L, et al. Versatile fabrication of liquid metal nano-ink based flexible electronic devices. Appl Mater Today 2021;22:100903.
21. Sun X, Cui B, Yuan B, et al. Liquid metal microparticles phase change medicated mechanical destruction for enhanced tumor cryoablation and dual-mode imaging. Adv Funct Mater 2020;30:2003359.
22. Gao Q, Li H, Zhang J, Xie Z, Zhang J, Wang L. Microchannel structural design for a room-temperature liquid metal based super-stretchable sensor. Sci Rep 2019;9:5908.
23. Gao Y, Ota H, Schaler EW, et al. Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring. Adv Mater 2017;29:1701985.
24. Chang H, Guo R, Sun Z, et al. Direct writing and repairable paper flexible electronics using nickel-liquid metal ink. Adv Mater Interfaces 2018;5:1800571.
25. Kim S, Oh J, Jeong D, Bae J. Direct wiring of eutectic gallium-indium to a metal electrode for soft sensor systems. ACS Appl Mater Interfaces 2019;11:20557-65.
26. Yoon Y, Kim S, Kim D, Kauh SK, Lee J. Four degrees-of-freedom direct writing of liquid metal patterns on uneven surfaces. Adv Mater Technol 2019;4:1800379.
27. Guo C, Yu Y, Liu J. Rapidly patterning conductive components on skin substrates as physiological testing devices via liquid metal spraying and pre-designed mask. J Mater Chem B 2014;2:5739-45.
28. Plevachuk Y, Sklyarchuk V, Shevchenko N, Eckert S. Electrophysical and structure-sensitive properties of liquid Ga-In alloys. Int J Mater Res 2015;106:66-71.
29. Plevachuk Y, Sklyarchuk V, Eckert S, Gerbeth G, Novakovic R. Thermophysical properties of the liquid Ga-In-Sn eutectic alloy. J Chem Eng Data 2014;59:757-63.
31. Cicco AD, Filipponi A. Local correlations in liquid and supercooled gallium probed by X-ray absorption spectroscopy. Europhys Lett 1994;27:407-12.
32. Tang S, Mitchell DR, Zhao Q, et al. Phase separation in liquid metal nanoparticles. Matter 2019;1:192-204.
33. Koster JN. Directional solidification and melting of eutectic GaIn. Cryst Res Technol 1999;34:1129-40. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1521-4079(199911)34:9%3C1129::AID-CRAT1129%3E3.0.CO;2-P. [Last accessed on 24 Aug 2023]
34. Chitambar CR. Medical applications and toxicities of gallium compounds. Int J Environ Res Public Health 2010;7:2337-61.
35. White SJO, Shine JP. Exposure potential and health impacts of indium and gallium, metals critical to emerging electronics and energy technologies. Curr Environ Health Rep 2016;3:459-67.
36. Li J, Guo C, Wang Z, Gao K, Shi X, Liu J. Electrical stimulation towards melanoma therapy via liquid metal printed electronics on skin. Clin Transl Med 2016;5:21.
37. Fan L, Duan M, Xie Z, et al. Injectable and radiopaque liquid metal/calcium alginate hydrogels for endovascular embolization and tumor embolotherapy. Small 2019;16:1903421.
38. Hallfors N, Khan A, Dickey MD, Taylor AM. Integration of pre-aligned liquid metal electrodes for neural stimulation within a user-friendly microfluidic platform. Lab Chip 2013;13:522-6.
39. Zhang M, Yao S, Rao W, Liu J. Transformable soft liquid metal micro/nanomaterials. Mate Sci Eng R Rep 2019;138:1-35.
40. Domingo JL, Corbella J. A review of the health hazards from gallium exposure. Trace Elem Med 1991;8:56-64. Available from: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=5304637. [Last accessed on 24 Aug 2023].
41. Liu S, Sun X, Kemme N, et al. Can liquid metal flow in microchannels made of its own oxide skin? Microfluid Nanofluid 2016;20:3.
42. Regan MJ, Tostmann H, Pershan PS, et al. X-ray study of the oxidation of liquid-gallium surfaces. Phys Rev B 1997;55:10786-90.
43. Cademartiri L, Thuo MM, Nijhuis CA, et al. Electrical resistance of AgTS-S(CH2)n-1CH3//Ga2O3/EGaIn tunneling junctions. J Phys Chem C 2012;116:10848-60.
44. Dickey MD. Emerging applications of liquid metals featuring surface oxides. ACS Appl Mater Interfaces 2014;6:18369-79.
45. Zhang Q, Gao Y, Liu J. Atomized spraying of liquid metal droplets on desired substrate surfaces as a generalized way for ubiquitous printed electronics. Appl Phys A 2014;116:1091-7.
46. Gao Y, Li H, Liu J. Direct writing of flexible electronics through room temperature liquid metal ink. PLoS One 2012;7:e45485.
47. Zheng Y, He ZZ, Yang J, Liu J. Personal electronics printing via tapping mode composite liquid metal ink delivery and adhesion mechanism. Sci Rep 2014;4:4588.
48. Tang L, Cheng S, Zhang L, et al. Printable metal-polymer conductors for highly stretchable bio-devices. iScience 2018;4:302-11.
49. Boley JW, White EL, Kramer RK. Mechanically sintered gallium-indium nanoparticles. Adv Mater 2015;27:2355-60.
50. Ren L, Zhuang J, Casillas G, et al. Nanodroplets for stretchable superconducting circuits. Adv Funct Mater 2016;26:8111-8.
51. Li X, Li M, Zong L, et al. Liquid metal droplets wrapped with polysaccharide microgel as biocompatible aqueous ink for flexible conductive devices. Adv Funct Mater 2018;28:1804197.
52. Li H, Qiao R, Davis TP, Tang SY. Biomedical applications of liquid metal nanoparticles: a critical review. Biosensors 2020;10:196.
53. Tang S, Qiao R. Liquid metal particles and polymers: a soft-soft system with exciting properties. Acc Mater Res 2021;2:966-78.
54. Lin Y, Cooper C, Wang M, Adams JJ, Genzer J, Dickey MD. Handwritten, soft circuit boards and antennas using liquid metal nanoparticles. Small 2015;11:6397-403.
55. Liu S, Yuen MC, White EL, et al. Laser sintering of liquid metal nanoparticles for scalable manufacturing of soft and flexible electronics. ACS Appl Mater Interfaces 2018;10:28232-41.
56. Deng B, Cheng GJ. Pulsed laser modulated shock transition from liquid metal nanoparticles to mechanically and thermally robust solid-liquid patterns. Adv Mater 2019;31:e1807811.
57. Li X, Li M, Xu J, You J, Yang Z, Li C. Evaporation-induced sintering of liquid metal droplets with biological nanofibrils for flexible conductivity and responsive actuation. Nat Commun 2019;10:3514.
58. Bartlett MD, Kazem N, Powell-Palm MJ, et al. High thermal conductivity in soft elastomers with elongated liquid metal inclusions. Proc Natl Acad Sci U S A 2017;114:2143-8.
59. Fassler A, Majidi C. Liquid-phase metal inclusions for a conductive polymer composite. Adv Mater 2015;27:1928-32.
60. Markvicka EJ, Bartlett MD, Huang X, Majidi C. An autonomously electrically self-healing liquid metal-elastomer composite for robust soft-matter robotics and electronics. Nat Mater 2018;17:618-24.
61. Ford MJ, Patel DK, Pan C, Bergbreiter S, Majidi C. Controlled assembly of liquid metal inclusions as a general approach for multifunctional composites. Adv Mater 2020;32:e2002929.
62. Tang L, Mou L, Zhang W, Jiang X. Large-scale fabrication of highly elastic conductors on a broad range of surfaces. ACS Appl Mater Interfaces 2019;11:7138-47.
63. Wang H, Yao Y, He Z, et al. A highly stretchable liquid metal polymer as reversible transitional insulator and conductor. Adv Mater 2019;31:e1901337.
64. Yun G, Tang S, Zhao Q, et al. Liquid metal composites with anisotropic and unconventional piezoconductivity. Matter 2020;3:824-41.
65. Yun G, Tang SY, Sun S, et al. Liquid metal-filled magnetorheological elastomer with positive piezoconductivity. Nat Commun 2019;10:1300.
66. Bartlett MD, Fassler A, Kazem N, Markvicka EJ, Mandal P, Majidi C. Liquid metals: stretchable, high-k dielectric elastomers through liquid-metal inclusions (Adv. Mater. 19/2016). Adv Mater 2016;28:3791.
67. Kazem N, Bartlett MD, Majidi C. Extreme toughening of soft materials with liquid metal. Adv Mater 2018;30:e1706594.
68. Pan C, Markvicka EJ, Malakooti MH, et al. A liquid-metal-elastomer nanocomposite for stretchable dielectric materials. Adv Mater 2019;31:1900663.
69. Kazem N, Hellebrekers T, Majidi C. Soft multifunctional composites and emulsions with liquid metals. Adv Mater 2017;29:1605985.
71. Chen X, Wan H, Guo R, et al. A double-layered liquid metal-based electrochemical sensing system on fabric as a wearable detector for glucose in sweat. Microsyst Nanoeng 2022;8:48.
72. Lin R, Kim HJ, Achavananthadith S, et al. Digitally-embroidered liquid metal electronic textiles for wearable wireless systems. Nat Commun 2022;13:2190.
73. Lee GH, Woo H, Yoon C, et al. A personalized electronic tattoo for healthcare realized by on-the-spot assembly of an intrinsically conductive and durable liquid-metal composite (Adv. Mater. 32/2022). Adv Mater 2022;34:2270236.
74. Yang Y, Han J, Huang J, et al. Stretchable energy-harvesting tactile interactive interface with liquid-metal-nanoparticle-based electrodes. Adv Funct Mater 2020;30:1909652.
75. Port A, Luechinger R, Albisetti L, et al. Detector clothes for MRI: a wearable array receiver based on liquid metal in elastic tubes. Sci Rep 2020;10:8844.
76. Gu L, Poddar S, Lin Y, et al. A biomimetic eye with a hemispherical perovskite nanowire array retina. Nature 2020;581:278-82.
77. Khondoker MAH, Ostashek A, Sameoto D. Direct 3D printing of stretchable circuits via liquid metal co-extrusion within thermoplastic filaments. Adv Eng Mater 2019;21:1900060.
78. Teng L, Ye S, Handschuh-wang S, Zhou X, Gan T, Zhou X. Liquid metal-based transient circuits for flexible and recyclable electronics. Adv Funct Mater 2019;29:1808739.
79. Chen Y, Liu Y, Ren J, et al. Conformable core-shell fiber tactile sensor by continuous tubular deposition modeling with water-based sacrificial coaxial writing. Mater Des 2020;190:108567.
80. Guo R, Tang J, Dong S, et al. One-step liquid metal transfer printing: toward fabrication of flexible electronics on wide range of substrates. Adv Mater Technol 2018;3:1800265.
81. Park TH, Kim J, Seo S. Facile and rapid method for fabricating liquid metal electrodes with highly precise patterns via one-step coating. Adv Funct Mater 2020;30:2003694.
82. Kim MG, Brown DK, Brand O. Nanofabrication for all-soft and high-density electronic devices based on liquid metal. Nat Commun 2020;11:1002.
83. Abbasi R, Mayyas M, Ghasemian MB, et al. Photolithography-enabled direct patterning of liquid metals. J Mater Chem C 2020;8:7805-11.
84. Ozutemiz KB, Wissman J, Ozdoganlar OB, Majidi C. EGaIn-metal interfacing for liquid metal circuitry and microelectronics integration. Adv Mater Interfaces 2018;5:1701596.
85. Xu J, Guo H, Ding H, et al. Printable and recyclable conductive ink based on a liquid metal with excellent surface wettability for flexible electronics. ACS Appl Mater Interfaces 2021;13:7443-52.
86. Silva CA, lv J, Yin L, et al. Liquid metal based island-bridge architectures for all printed stretchable electrochemical devices. Adv Funct Mater 2020;30:2002041.
87. Zhou L, Fu J, Gao Q, Zhao P, He Y. All-printed flexible and stretchable electronics with pressing or freezing activatable liquid-metal-silicone inks. Adv Funct Mater 2020;30:1906683.
88. Guo R, Wang H, Sun X, et al. Semiliquid metal enabled highly conductive wearable electronics for smart fabrics. ACS Appl Mater Interfaces 2019;11:30019-27.
89. Guo R, Cui B, Zhao X, et al. Cu-EGaIn enabled stretchable e-skin for interactive electronics and CT assistant localization. Mater Horiz 2020;7:1845-53.
90. Wang J, Cai G, Li S, Gao D, Xiong J, Lee PS. Printable superelastic conductors with extreme stretchability and robust cycling endurance enabled by liquid-metal particles. Adv Mater 2018;30:e1706157.
91. Guo R, Wang X, Chang H, et al. Ni-GaIn amalgams enabled rapid and customizable fabrication of wearable and wireless healthcare electronics. Adv Eng Mater 2018;20:1800054.
92. Dong C, Leber A, Das Gupta T, et al. High-efficiency super-elastic liquid metal based triboelectric fibers and textiles. Nat Commun 2020;11:3537.
93. Zhang X, Ai J, Zou R, Su B. Compressible and stretchable magnetoelectric sensors based on liquid metals for highly sensitive, self-powered respiratory monitoring. ACS Appl Mater Interfaces 2021;13:15727-37.
94. Feng B, Jiang X, Zou G, et al. Nacre-inspired, liquid metal-based ultrasensitive electronic skin by spatially regulated cracking strategy. Adv Funct Mater 2021;31:2102359.
95. Mengüç Y, Park Y, Pei H, et al. Wearable soft sensing suit for human gait measurement. Int J Rob Res 2014;33:1748-64.
96. Do TN, Phan H, Nguyen T, Visell Y. Miniature soft electromagnetic actuators for robotic applications. Adv Funct Mater 2018;28:1800244.
97. Xu C, Ma B, Yuan S, Zhao C, Liu H. High-resolution patterning of liquid metal on hydrogel for flexible, stretchable, and self-healing electronics. Adv Electron Mater 2020;6:1900721.
98. Wissman JP, Sampath K, Freeman SE, Rohde CA. Capacitive bio-inspired flow sensing cupula. Sensors 2019;19:2639.
99. Zhang L, Gao M, Wang R, Deng Z, Gui L. Stretchable pressure sensor with leakage-free liquid-metal electrodes. Sensors 2019;19:1316.
100. Won D, Baek S, Kim H, Kim J. Arrayed-type touch sensor using micro liquid metal droplets with large dynamic range and high sensitivity. Sens Actuator A Phys 2015;235:151-7.
101. Won D, Baek S, Huh M, Kim H, Lee S, Kim J. Robust capacitive touch sensor using liquid metal droplets with large dynamic range. Sensor Actuat A Phys 2017;259:105-11.
102. Yeo JC, Kenry, Yu J, Loh KP, Wang Z, Lim CT. Triple-state liquid-based microfluidic tactile sensor with high flexibility, durability, and sensitivity. ACS Sens 2016;1:543-51.
103. Kim K, Choi J, Jeong Y, et al. Wearable sensors: highly sensitive and wearable liquid metal-based pressure sensor for health monitoring applications: integration of a 3D-printed microbump array with the microchannel. Adv Healthc Mater 2019;8:1900986.
104. Yeo JC, Yu J, Koh ZM, Wang Z, Lim CT. Wearable tactile sensor based on flexible microfluidics. Lab Chip 2016;16:3244-50.
105. Jeong YR, Kim J, Xie Z, et al. A skin-attachable, stretchable integrated system based on liquid GaInSn for wireless human motion monitoring with multi-site sensing capabilities. NPG Asia Mater 2017;9:e443.
106. Park Y, Majidi C, Kramer R, Bérard P, Wood RJ. Hyperelastic pressure sensing with a liquid-embedded elastomer. J Micromech Microeng 2010;20:125029.
107. Zhang M, Wang X, Huang Z, Rao W. Liquid metal based flexible and implantable biosensors. Biosensors 2020;10:170.
108. Tepáyotl-ramírez D, Lu T, Park Y, Majidi C. Collapse of triangular channels in a soft elastomer. Appl Phys Lett 2013;102:044102.
109. Nan K, Babaee S, Chan WW, et al. Low-cost gastrointestinal manometry via silicone-liquid-metal pressure transducers resembling a quipu. Nat Biomed Eng 2022;6:1092-104.
110. Zhu M, Wang Y, Lou M, Yu J, Li Z, Ding B. Bioinspired transparent and antibacterial electronic skin for sensitive tactile sensing. Nano Energy 2021;81:105669.
111. Lin X, Mao Y, Li P, et al. Ultra-conformable ionic skin with multi-modal sensing, broad-spectrum antimicrobial and regenerative capabilities for smart and expedited wound care. Adv Sci 2021;8:2004627.
112. Jiang C, Gao K, Zhao N, et al. A wearable braille recognition system based on high density tactile sensors. In: 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS); 2020 Jan 18-22; Vancouver, Canada; IEEE; 2020. p. 28-31.
113. Leber A, Dong C, Chandran R, Das Gupta T, Bartolomei N, Sorin F. Soft and stretchable liquid metal transmission lines as distributed probes of multimodal deformations. Nat Electron 2020;3:316-26.
114. Kim S, Oh J, Jeong D, Park W, Bae J. Consistent and reproducible direct ink writing of eutectic gallium-indium for high-quality soft sensors. Soft Robot 2018;5:601-12.
115. Wu Y, Zhen R, Liu H, et al. Liquid metal fiber composed of a tubular channel as a high-performance strain sensor. J Mater Chem C 2017;5:12483-91.
116. Lu T, Wissman J, Ruthika, Majidi C. Soft anisotropic conductors as electric vias for Ga-based liquid metal circuits. ACS Appl Mater Interfaces 2015;7:26923-9.
117. So J, Thelen J, Qusba A, Hayes GJ, Lazzi G, Dickey MD. Reversibly deformable and mechanically tunable fluidic antennas. Adv Funct Mater 2009;19:3632-7.
118. Tang L, Shang J, Jiang X. Multilayered electronic transfer tattoo that can enable the crease amplification effect. Sci Adv 2021;7:eabe3778.
119. Kramer RK, Majidi C, Sahai R, Wood RJ. Soft curvature sensors for joint angle proprioception. In: 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems; 2011 Sep 25-30; San Francisco, CA, USA. IEEE; 2011.
120. Li G, Zhang M, Liu S, et al. Three-dimensional flexible electronics using solidified liquid metal with regulated plasticity. Nat Electron 2023;6:154-63.
121. Uchida K, Takahashi S, Harii K, et al. Observation of the spin Seebeck effect. Nature 2008;455:778-81.
122. Adachi H, Uchida K, Saitoh E, Maekawa S. Theory of the spin Seebeck effect. Rep Prog Phys 2013;76:036501.
123. Li H, Yang Y, Liu J. Printable tiny thermocouple by liquid metal gallium and its matching metal. Appl Phys Lett 2012;101:073511.
124. Wang Q, Gao M, Zhang L, Deng Z, Gui L. A handy flexible micro-thermocouple using low-melting-point metal alloys. Sensors 2019;19:314.
125. Yu Y, Zhang J, Liu J. Biomedical implementation of liquid metal ink as drawable ECG electrode and skin circuit. PLoS One 2013;8:e58771.
126. Guo R, Sun X, Yao S, et al. Semi-liquid-metal-(Ni-EGaIn)-based ultraconformable electronic tattoo. Adv Mater Technol 2019;4:1900183.
127. Timosina V, Cole T, Lu H, et al. A non-newtonian liquid metal enabled enhanced electrography. Biosens Bioelectron 2023;235:115414.
128. Ding L, Hang C, Cheng S, et al. A soft, conductive external stent inhibits intimal hyperplasia in vein grafts by electroporation and mechanical restriction. ACS Nano 2020;14:16770-80.
130. Liu F, Yu Y, Yi L, Liu J. Liquid metal as reconnection agent for peripheral nerve injury. Science Bulletin 2016;61:939-47.
131. Dong R, Wang L, Hang C, et al. Printed stretchable liquid metal electrode arrays for in vivo neural recording. Small 2021;17:e2006612.
132. Wen X, Wang B, Huang S, et al. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosens Bioelectron 2019;131:37-45.
133. Lim T, Kim M, Akbarian A, Kim J, Tresco PA, Zhang H. Conductive polymer enabled biostable liquid metal electrodes for bioelectronic applications. Adv Healthc Mater 2022;11:e2102382.
134. Wang S, Nie Y, Zhu H, et al. Intrinsically stretchable electronics with ultrahigh deformability to monitor dynamically moving organs. Sci Adv 2022;8:eabl5511.
135. Li G, Ma X, Xu Z, et al. A crack compensation strategy for highly stretchable conductors based on liquid metal inclusions. iScience 2022;25:105495.
136. Schedle A, Samorapoompichit P, Rausch-Fan XH, et al. Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. J Dent Res 1995;74:1513-20.
137. Kim JH, Kim S, So JH, Kim K, Koo HJ. Cytotoxicity of gallium-indium liquid metal in an aqueous environment. ACS Appl Mater Interfaces 2018;10:17448-54.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Li G, Liu S, Xu Z, Guo J, Tang SY, Ma X. Recent advancements in liquid metal enabled flexible and wearable biosensors. Soft Sci 2023;3:37. http://dx.doi.org/10.20517/ss.2023.30
AMA Style
Li G, Liu S, Xu Z, Guo J, Tang SY, Ma X. Recent advancements in liquid metal enabled flexible and wearable biosensors. Soft Science. 2023; 3(4): 37. http://dx.doi.org/10.20517/ss.2023.30
Chicago/Turabian Style
Li, Guoqiang, Sanhu Liu, Zhiwu Xu, Jinhong Guo, Shi-Yang Tang, Xing Ma. 2023. "Recent advancements in liquid metal enabled flexible and wearable biosensors" Soft Science. 3, no.4: 37. http://dx.doi.org/10.20517/ss.2023.30
ACS Style
Li, G.; Liu S.; Xu Z.; Guo J.; Tang S.Y.; Ma X. Recent advancements in liquid metal enabled flexible and wearable biosensors. Soft. Sci. 2023, 3, 37. http://dx.doi.org/10.20517/ss.2023.30
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.