Wearable plasmonic biofluid sensors as your photonic skin
Abstract
Noninvasive monitoring of markers in biofluids is of paramount significance for health and welfare, which is being integrated into the next-generation consumable wearables. Movement-free sweat extraction and continuous monitoring of fresh sweat are two major challenges for wearable plasmonic sweat sensors. In this perspective, we highlight recent approaches that integrated an electronic sweat extraction system and a microfluidic system with plasmonic sensors to address the challenge. The future directions of systematic integration and miniaturization are discussed.
Keywords
Wearable sensors that allow the recognition of molecular-level traces in skin-accessible biofluids are considered as a promising technology with great commercial potential[1,2]. Existing sensing modalities mainly rely on electrochemical and colorimetric approaches[3,4]. These sensors mainly rely on enzymes and/or antibodies as biorecognition elements to achieve specific quantification of biomarkers. However, enzymes and antibodies are prone to degrade over time and lose their functionality after multiple uses[5]. Additionally, the exudation of most biofluids is too small to be consecutively collected[6]. Therefore, continuous measurements of analytes in biofluids with high sensitivity, selectivity, and environmental stability remain challenging.
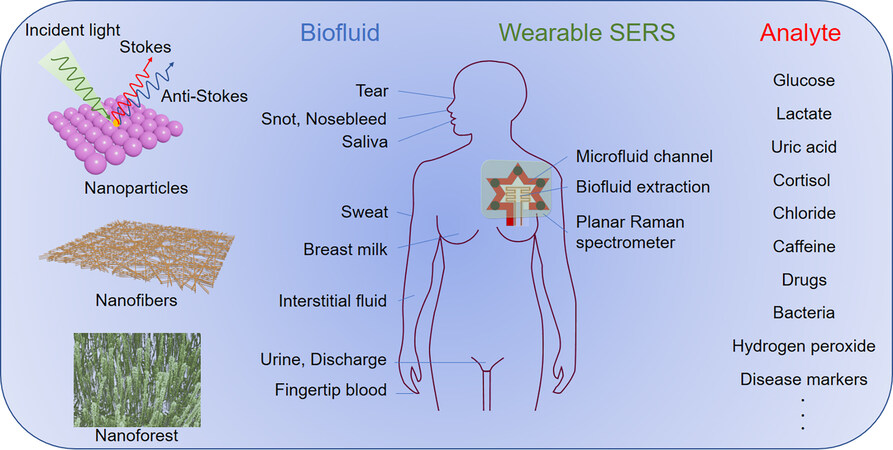
Surface-enhanced Raman spectroscopy (SERS) technology offers unique sensitivity, selectivity, and robustness compared with alternative methods. As shown in Figure 1, plasmonic materials typically consist of metallic nanostructures that support electromagnetic oscillations and electron transfer at their interfaces, corresponding to electromagnetic and chemical enhancement, respectively[7]. Metallic nanoparticle arrays with good performance are widely used as SERS substrates, but their poor mechanical durability poses challenges for wearable sensors[8]. Other nanostructures, like nanofiber and nanoforest, exhibit good mechanical flexibility and even stretchability, but their uniformity and enhancement factor should be further improved[1,9,10]. Regarding biomarkers detection by SERS, it generally relies on Stokes Raman scattering[11]. The magnitude of the wavelength shift depends on the molecular vibrational state, so measuring the light emitted by a sample upon excitation provides a way to identify the molecular bonds, thereby identifying the molecular composition of the sample and/or discriminating different biomarkers in the sample[12].
Figure 1. Schematic of next-generation wearable plasmonic biosensors for continuous monitoring analytes in human biofluids. The microfluidic system and biofluid extraction system are integrated with planar Raman spectrometer. The typical plasmonic surfaces and various analytes in different biofluids are also given.
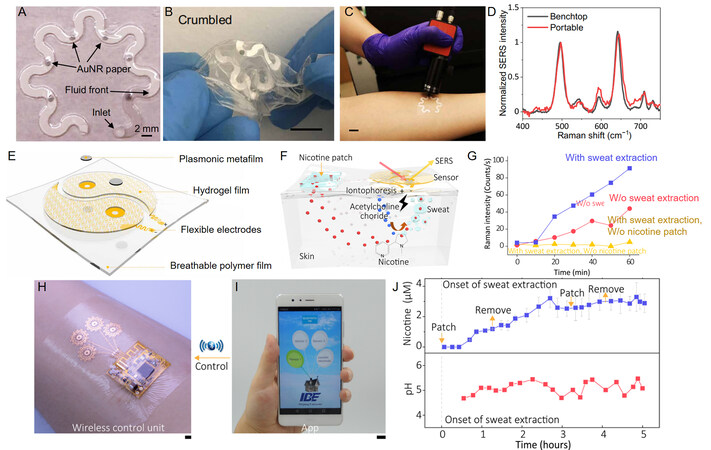
Mogera et al. from Texas A&M University introduce a wearable plasmonic paper-based microfluidic (called paperfluidic) system for continuous and simultaneous quantitative analysis of sweat loss, sweat rate, and metabolites in sweat[13]. The wearable plasmonic sweat sensor comprises five functional layers, including a double-sided adhesive for a robust interface, carbon black as a laser blocker, cellulose chromatography paper with a serpentine design as a microfluidic channel, a plasmonic layer, as well as top polydimethylsiloxane encapsulation film [Figure 2A]. The chromatography-grade filter adsorbed with gold nanorods is used as plasmonic surface. To overlap with the laser excitation wavelength, the optimized gold nanorods with 56.9 nm in length and 14.5 nm in diameter were synthesized by seed-mediated method. The resulting sensor is stretchable and no delamination can be observed between all functional layers under 30% stretch. Also, the SERS intensity change of uric acid is only 9% under 30% stretch compared to that unstretched. Crumbled paperfluidic device further demonstrates its excellent mechanical robustness [Figure 2B]. The wicking rate for sweat is quantified to be 232 µL cm2 h-1 for the paperfluidic device with a channel width of 2 mm, which is much higher than the typical sweat rate of 12 to 120 µL cm2 h-1. Furthermore, the point-of-care wearable plasmonic paperfluidic device is evaluated using a portable Raman spectrometer [Figure 2C]. After 20 min running, the sweat volume collected by the device on the forearm of a healthy human subject is 13.7 µL and the quantified concentration of uric acid in sweat is 28 µM, which is consistent with that measured by benchtop one [Figure 2D]. Other biomarkers in sweat, such as lactate and pH, can also be monitored in situ by integrating polydimethylsiloxane microfluidic with Ag nanoparticles as SERS surfaces[14].
Figure 2. Wearable SERS devices integrated with microfluid systems or sweat extraction systems. (A) A photo of an integrated paperfluidic device with six plasmonic sensors. (B) A photo of crumbled paperfluidic device. (C) A photo of wearable paperfluidic SERS device during the test with a portable Raman spectrometer. (D) Comparison of SERS spectra collected with benchtop and portable spectrometers. (E) Schematic structure of wearable SERS devices with sweat extraction system. (F) Schematic illustration showing the working principle of the sweat extraction system. (G) Evolution of the characteristic Raman peak of nicotine under different conditions. (H) A photo of the integrated SERS sensor array connected to a flexible stimulation circuit, which is mounted on human skin (I) A photo of the custom-developed control app for electrical stimulation. (J) Simultaneous tracking of pH and nicotine concentration. (A-D) are adapted with permission. Copyright 2022, AAAS. (E-J) are adapted with permission. Copyright 2022, AAAS.
Since most of us prefer staying in a comfortable environment, the sweating amount is too low to be accurately monitored continuously. In addition to sweat extraction through hot air temperature, physical activity, and nerve stimulation, iontophoresis is also an effective method[15]. Wang et al. present a wearable plasmonic-electronic integrated sensor by combining plasmonic metasurfaces with a flexible electronic sweat extraction system [Figure 2E][16]. Such an integrated sensor enables noninvasive sweat extraction and detection of trace metabolized drugs without intentional physical activity. The sweat samples containing various drugs (lidocaine, methotrexate, cocaine and nicotine) exhibited entirely different SERS spectra. Also, real-time tracking of drug concentrations in sweat provides an individual drug metabolism profile.
The integrated sensor leverages the authors’ expertise in microfabrication of wearable electronics. Specifically, spiral Cu mesh electrodes are first fabricated by laser direct writing of a 6-µm thick Cu film, which can operate normally under ~50% strain after transferring onto elastomer substrate. Then, a 100-µm thick hydrogel film containing 10% acetylcholine chloride is transferred onto the electrodes. After that, a metafilm of silver nanocubes assembled by Langmuir Blodgett is mouthed into through-hole cutouts at the centers of the electrodes. The sweat extraction is based on an iontophoresis process of acetylcholine chloride into secretory sweat glands by applying a 5-mA current for 5 min, which is widely used in clinical noninvasive sweat sampling. The SERS intensity remains stable under ~30% strain even after 1000 stretching-relaxation cycles. To access the individuals’ drug delivery, uptake, and metabolic rate, a nicotine patch containing approximately 10 mg of nicotine is placed near the plasmonic sensor [Figure 2F]. Then, the Raman intensity of nicotine is recorded every 10 min with or without sweat extraction. Results show that the SERS spectrum of nicotine was observed in the sweat after electrical stimulation for ~20 min. In contrast, the SERS spectrum of nicotine could barely be observed after ~40 min and the signal intensities remained much lower compared to the above results without iontophoresis for sweat extraction [Figure 2G]. The team further presents a plasmonic sensor array integrated with a flexible electrical stimulation circuit for tracking both nicotine and pH simultaneously [Figure 2H and I]. A continued increase of nicotine concentration is observed whether the patch is attached or removed, while the pH value in sweat fluctuates between 4.8 and 5.5 during 5 h test [Figure 2J].
In summary, the integration of SERS devices with microfluid systems and biofluid extraction systems are effective approaches to achieve continuous real-time monitoring of sweat. The microfluidic channel permits sweat handling in a regulated and high temporal-resolution manner, allowing for refreshable SERS evaluation. Meanwhile, the electrical stimulation facilities sweat production with enough amount without intense physical activity. However, mutual interferences may exist that affect the accuracy of this technology. For instance, the fresh sweat in microfluid channel may not be able to remove the absorbed biomarkers, and electrical stimulation may change the concentrations of biomarkers. Thus, future considerations may involve (1) the systematic integration with careful device and layout design; (2) optimal discharge of biofluid after use; (3) breathable device design to avoid skin inflammation and bacterial growth; and (4) miniaturization of Raman spectrometer with use of planar optics[17]. With these advances, the integrated wearable and breathable SERS devices will achieve the in-situ collection, storage, analysis and discharge of biofluid, which will be of great significance for health monitoring and diagnosis.
DECLARATIONS
Authors’ contributionsWrote the original draft: Liu L
Supervised, reviewed and revised the manuscript: Wang B, Ahn JH
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported in part by the Natural Science Foundation of Jiangsu Province under Grant No. BK20220815 and in part by the Program of Jiangsu Specially-Appointed Professor.
Conflicts of interestThe authors declare no competing interests.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Liu L, Martinez Pancorbo P, Xiao T, et al. Highly scalable, wearable surface-enhanced raman spectroscopy. Adv Opt Mater 2022;10:2200054.
2. Koh EH, Lee WC, Choi YJ, et al. A wearable surface-enhanced raman scattering sensor for label-free molecular detection. ACS Appl Mater Interfaces 2021;13:3024-32.
3. Koh A, Kang D, Xue Y, et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Transl Med 2016;8:366ra165.
4. Patel S, Ershad F, Zhao M, et al. Wearable electronics for skin wound monitoring and healing. Soft Sci 2022;2:9.
5. Bandodkar AJ, Imani S, Nuñez-Flores R, et al. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosens Bioelectron 2018;101:181-7.
6. Pu Z, Zhang X, Yu H, et al. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring. Sci Adv 2021;7:eabd0199.
7. Langer J, Jimenez de Aberasturi D, Aizpurua J, et al. Present and future of surface-enhanced raman scattering. ACS Nano 2020;14:28-117.
8. Xie L, Zeng H, Zhu J, et al. State of the art in flexible SERS sensors toward label-free and onsite detection: from design to applications. Nano Res 2022;15:4374-94.
9. Chen N, Xiao TH, Luo Z, et al. Porous carbon nanowire array for surface-enhanced Raman spectroscopy. Nat Commun 2020;11:4772.
10. Yang Y, Peng Y, Lin C, et al. Human ACE2-Functionalized gold “Virus-Trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nanomicro Lett 2021;13:109.
12. Lee KS, Landry Z, Pereira FC, et al. Raman microspectroscopy for microbiology. Nat Rev Methods Primers 2021;1:80.
13. Mogera U, Guo H, Namkoong M, Rahman MS, Nguyen T, Tian L. Wearable plasmonic paper-based microfluidics for continuous sweat analysis. Sci Adv 2022;8:eabn1736.
14. He X, Fan C, Luo Y, Xu T, Zhang X. Flexible microfluidic nanoplasmonic sensors for refreshable and portable recognition of sweat biochemical fingerprint. NPJ Flex Electron 2022;6:60.
15. Sempionatto JR, Jeerapan I, Krishnan S, Wang J. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal Chem 2020;92:378-96.
16. Wang Y, Zhao C, Wang J, et al. Wearable plasmonic-metasurface sensor for noninvasive and universal molecular fingerprint detection on biointerfaces. Sci Adv 2021;7:eabe4553.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Liu L, Ahn JH, Wang B. Wearable plasmonic biofluid sensors as your photonic skin. Soft Sci 2023;3:6. http://dx.doi.org/10.20517/ss.2022.31
AMA Style
Liu L, Ahn JH, Wang B. Wearable plasmonic biofluid sensors as your photonic skin. Soft Science. 2023; 3(1): 6. http://dx.doi.org/10.20517/ss.2022.31
Chicago/Turabian Style
Liu, Limei, Jae-Hyuk Ahn, Binghao Wang. 2023. "Wearable plasmonic biofluid sensors as your photonic skin" Soft Science. 3, no.1: 6. http://dx.doi.org/10.20517/ss.2022.31
ACS Style
Liu, L.; Ahn J.H.; Wang B. Wearable plasmonic biofluid sensors as your photonic skin. Soft. Sci. 2023, 3, 6. http://dx.doi.org/10.20517/ss.2022.31
About This Article
Copyright
Data & Comments
Data

 Cite This Article 20 clicks
Cite This Article 20 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.