Crosslinked organosulfur-based self-assembled monolayers: formation and applications
Abstract
Self-assembled monolayers (SAMs) have found use in diverse applications that range from corrosion prevention to biosensing. However, for all of these applications, stability remains a key challenge for the utilization of SAMs. Over the last decade, intermolecular crosslinking as a method to enhance the thermal and chemical stability of SAMs has attracted increased attention from scientists and engineers. As such, this review introduces a variety of crosslinked SAMs: (1) aromatic thiol-based SAMs; (2) olefinic- and acetylenic-based alkanethiols; (3) other aliphatic alkanethiols; (4) silane-based alkanethiols; (5) boronic acid-based alkanethiols; and (6) crosslinked SAMs realized by hydrogen bonding. By offering insight into the structure-application relationships of the aforementioned SAMs, this review seeks to inspire researchers toward the development of new classes of SAMs with enhanced stabilities and working lifetimes.
Keywords
INTRODUCTION
The discovery of the spontaneous adsorption of organic alkane disulfides to metal substrates, such as gold, in 1983 by Nuzzo and Allara as the first example of Self-assembled monolayers (SAMs) and the subsequent use of alkanethiols has led to the widespread use of SAMs[1-3]. Over the past few decades, SAMs have been widely used as a robust surface modification method in various industrial fields, such as in microelectromechanical system (MEMS) devices[4], antifouling coatings for biomaterials[5], and microcontact printing[6,7]. Compared to conventional polymer coatings, such as polytetrafluoroethylene, SAMs applied onto a surface can be controlled to reach thicknesses in the nanometer range with high uniformity and hydrophobicity[8,9]. For example, silane-based SAMs are routinely applied as nanocoatings in MEMS devices due to their ability to decrease adhesion and friction leading to enhanced performance[10-13].
Compared to normal silane SAMs, which only bind to metal oxides and silicon, thiol-based adsorbates are more compatible with the surfaces of metals, such as gold, silver, and copper, or III-V compounds such as GaAs[14]. As a well-known system, stability has been the major issue that has hindered thiol-based SAMs for further applications. Compared to N-heterocyclic carbenes (NHC)-based SAMs on gold or silane-based SAMs on silicon, which form strong covalent bonds between the adsorbates and substrates, the S-Au interaction is less stable in ambient conditions[15,16]. Previous research has shown that normal alkanethiol SAMs organized by van der Waals (vdW) interaction are sensitive to temperature and desorb from gold surfaces at 70 °C[2,17]. In addition, strong oxidizing reagents damage thin films and produce disulfides in a short period of time.
To broaden the application of SAMs, researchers have put in great effort to develop a more stable SAM system on gold that shows good resistance to high temperatures and harsh chemical conditions. Intermolecular crosslinking is an effective method used to enhance the thermal and chemical stability of SAMs. It is noted that SAM molecules consist of three key parts: the headgroup binding to the corresponding substrate; the methylene spacer, which provides vdW interchain interactions; and the tailgroup[18]. Theoretically, intermolecular crosslinking can occur in all three parts of the SAM molecules. However, unlike the natural formation of a polymerized network for the silane headgroup, the bonding nature of sulfur atoms of thiols determines that crosslinking at the headgroup for thiol-based SAMs is not achievable[19,20].
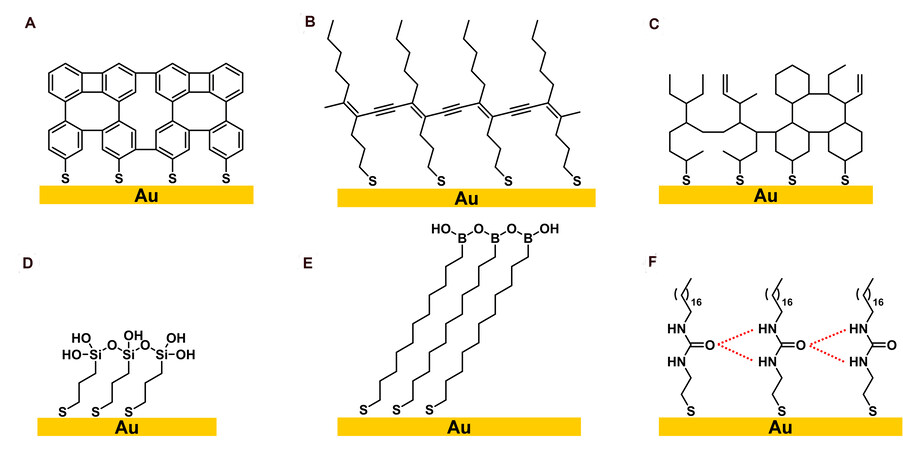
This review focuses on the crosslinking process of corresponding adsorbates and their applications in materials science. The crosslinked SAMs introduced in this chapter are divided into six categories: (1) aromatic thiol-based SAMs; (2) olefinic- and acetylenic-based alkanethiols; (3) other aliphatic alkanethiols; (4) silane-based alkanethiols; (5) boronic acid-based alkanethiols; and (6) crosslinked SAMs realized by hydrogen bonding [Figure 1].
Figure 1. Six different types of crosslinked SAMs: (A) aromatic thiol-based SAMs; (B) acetylenic-based alkanethiols; (C) other aliphatic alkanethiols; (D) silane-based alkanethiols; (E) boronic acid-based alkanethiols; and (F) crosslinked SAMs realized by hydrogen bonding. SAMs: Self-assembled monolayers.
Table 1 highlights six types of crosslinked SAMs, their corresponding methods of formation, and their potential applications. In contrast to previously published reviews of SAMs, which are largely focused on SAM structures, characterizations, and targeted applications, this review focuses on the stability of crosslinked SAMs and their corresponding applications in materials science. We hope this review will enlighten future advances in nanofabrication and bioengineering.
Crosslinked SAMs, their methods of formation, and their potential applications
| Crosslinked SAMs | Formation method | Applications |
| Aromatic thiol-based SAMs | Electron irradiation | 1. Electron-beam lithography 2. Surface functionalization 3. Surface-initiated polymerization 4. Carbon nanomembranes (CNMS) |
| Acetylenic-based alkanethiols | UV irradiation | 1. Photolithography 2. Nanoparticles for antifouling 3. Single-layer polydiacetylene fabrication |
| Aliphatic alkanethiols | Electron irradiation | 1. Electron-beam lithography |
| Silane-based alkanethiols | Hydrolysis | 1. Surface-initiated polymerization 2. Surface passivation |
| Boronic acid-based alkanethiols | Condensation in dry solvents | In development |
| Crosslinked SAMs realized by hydrogen bonding | Hydrogen bonding | 1. Selective binding of cations on surfaces 2. Thermally stable coatings |
CROSSLINKED AROMATIC THIOL-BASED SAMS
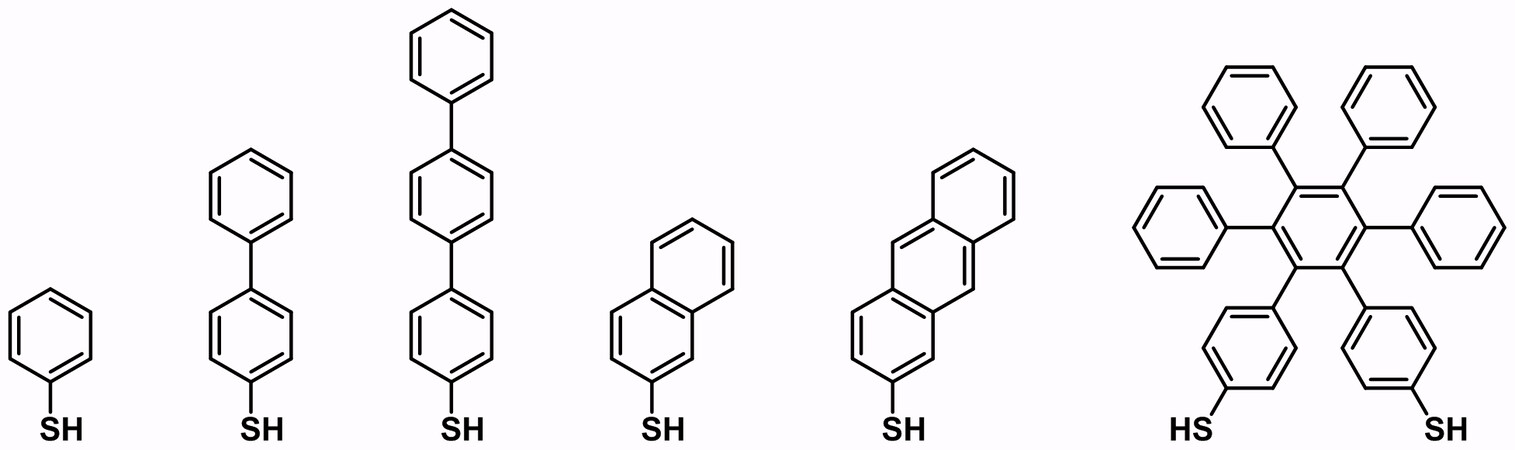
In 1999, Geyer et al.[21] discovered that biphenyl-4-thiol (BPT) can crosslink laterally upon low energy electron irradiation at 50 eV. Additionally, various kinds of SAMs based on aromatic thiols or thiolates can also undergo similar intermolecular crosslinking, which turns the original system into a crosslinked aromatic SAM system [Figure 2][22].
Figure 2. Structures of the precursors used for crosslinked aromatic thiol-based SAMs. Adapted from Ref.[22], copyright 2013 American Chemical Society. SAMs: Self-assembled monolayers.
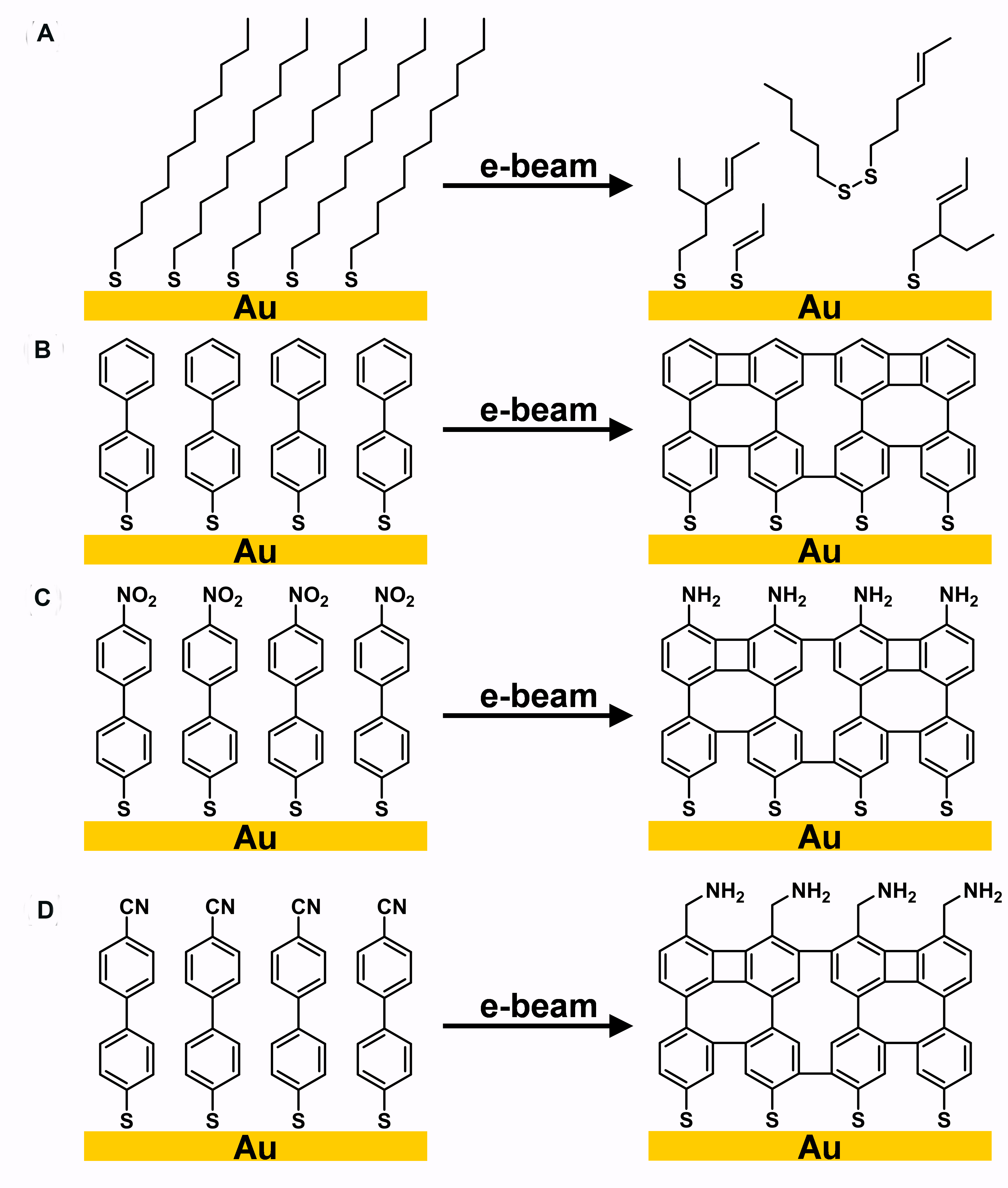
Figure 3[23] summarizes electron-induced crosslinking of aromatic thiol-based SAMs with three different tailgroups compared to n-alkanethiol-based SAMs. Unlike the BPT SAMs, which form a crosslinked network under electron irradiation [Figure 3B], normal alkanethiol-based SAMs partially desorb from gold surfaces and form double bonds randomly, as shown in Figure 3A[24,25]. Figure 3C shows that compared to hydrocarbon aromatic SAMs, nitro-terminated aromatic SAMs not only show crosslinking upon electron irradiation but also have their nitro groups reduced to amino groups[26]. In addition, Zharnikov and
Figure 3. Electron induced crosslinking of (A) aliphatic SAMs; (B) aromatic SAMs; (C) nitro-terminated aromatic SAMs; (D) nitrile-terminated aromatic SAMs. Adapted from Ref.[23], copyright 2012 American Chemical Society. SAMs: Self-assembled monolayers.
Taking BPT SAM as an example, the formation of the crosslinking can be summarized as a dissociative electron attachment (DEA) process [Figure 4][28]. First, under the impact of irradiation, such as electrons or X-rays, a gold substrate emits secondary electrons, as shown in Figure 4A and B[29]. The dissociative primary and secondary electrons lead to C-H bond cleavage of phenyl rings with hydrogen atoms left behind, which yields transient negative ions, as shown in Figure 4C[30]. In this process, the electron affinity of the phenyl radical is 1.1 eV, which is higher than that of hydrogen (0.75 eV)[31]. Thus, the dissociative electron attaches to the phenyl fragment ion instead of hydrogen. The DEA process for benzene is expressed in Equation 1.

The key factor for the lateral crosslinked product is the stability of the aromatic backbones, leading to the formation of single and double bonds between the phenyl rings, followed by the release of hydrogen. At the end of the reaction, the lateral crosslinking is terminated by the self-quenching of electron tunneling to the gold surface [Figure 4D][28]. In addition, researchers found that crosslinking of aromatic SAMs also occurs upon exposure to other sources of irradiation such as X-rays (10-100 eV)[32], helium ion beam (HIM, 35 keV)[33], and extreme UV (EUV, 92.5 eV)[34]. These results further confirmed that primary electrons originating from the incoming electron beam are not necessary and that, instead, secondary electrons emitted from the gold surface play a more important role in the crosslinking process.
Figure 4. Schematic representation of the different steps involved in the crosslinking of aromatic SAMs: (A) irradiation; (B) emission of secondary electrons; (C) dissociation of C-H bonds; and (D) self-quenching. Reprinted with permission from Ref.[28], copyright 2009 American Chemical Society. SAMs: Self-assembled monolayers.
In addition to irradiation energy, another key parameter for crosslinking aromatic SAMs is the electron dose, which is used to measure the quantity of charge per unit area upon irradiation. Interestingly, there is only a slight difference in crosslinking conditions (electron energy and dose) between extensive SAM precursors. To determine the optimal conditions for irradiation-induced crosslinking, scientists have used the cross section, σ, as a parameter to gauge when a rate reaches saturation behavior[35]. The cross section for the electron irradiation involves a balance between the crosslinking degree of the SAMs and the damage to the gold interface, which is determined as the primary electron energy of 50 eV[30]. Recently, Koch and
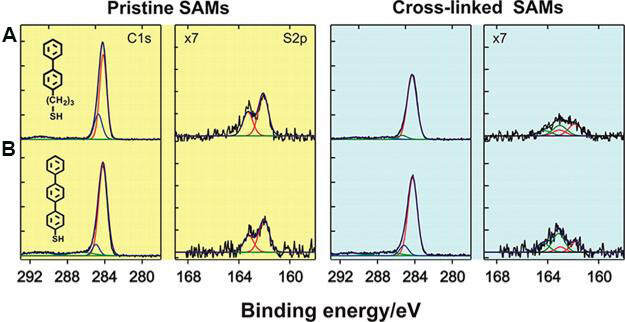
Compared to the pristine BPT SAM, infrared (IR) spectra for the crosslinked product show that two peaks arising from the C-H stretching vibration of the pristine phenyl ring at 3046 cm-1 and 3038 cm-1 disappear after electron irradiation. Figure 5 shows the XPS spectra for the C 1s and S 2p regions of (A) biphenyl-terminated SAMs and (B) terphenyl thiol SAMs following electron irradiation (50 eV, 60 mC/cm2)[22]. In the C 1s region, the integrated C 1s peak decreases in both of the SAMs investigated after crosslinking. The carbon content in the biphenyl-terminated SAM with hydrocarbon chains is decreased by 16%, and the calculated thickness is decreased from 12 Å to 10 Å, as determined by XPS. To explain these results, Schnack and co-workers performed a molecular dynamics simulation for crosslinking various biphenyl-terminated SAMs, which indicated that partial dissociation of the aromatic rings takes place to maximize the 2D molecular network[38]. In the XPS spectra obtained for S 2p, pristine SAMs exhibit a characteristic peak due to bound thiols (red lines), which appears as a doublet (S 2p3/2 and S 2p1/2) at binding energies of 162.0 eV and 163.0 eV, respectively[39]. The new peak appearing at 163.5 eV for the crosslinked SAMs suggests the formation of unbound sulfur species or disulfides because of the cleavage of S-Au bonds after irradiation, as shown by the green line obtained by spectral deconvolution. The results obtained from low-energy electron diffraction and a scanning tunneling microscope (STM) also indicate a loss of long-range order in the crosslinked aromatic SAMs.
Figure 5. XPS data for pristine and crosslinked SAMs. XPS spectra for C 1s and S 2p regions of pristine (in yellow) and electron irradiated (50 eV, 60 mC/cm2) monolayers (in blue) of (A) biphenyl-terminated SAM, (B) terphenyl thiol SAM. Adapted from Ref.[22], copyright 2013 American Chemical Society. XPS: X-ray photoelectron spectroscopy; SAM: self-assembled monolayer.
Applications
Electron-beam lithography
In the modern semiconductor industry, lithography is one of the basic processes used for transferring a designed pattern onto semiconductor substrates, followed by an etching or metal deposition process to fabricate microelectronic devices[40]. Mainstream lithography techniques include photolithography, electron-beam (e-beam) lithography, and ion-beam lithography[41]. In the case of e-beam lithography, surface patterning is achieved by directly controlling an electron beam to write onto a resist-coated surface[42]. There are two types of e-beam resists in e-beam lithography: positive and negative. With a positive resist, the areas exposed to the e-beam are removed by a developer solution in the next step to expose the bare substrate. In contrast, for a negative resist, only the areas of the resist exposed to the e-beam remain, while the other areas are removed[43]. To fabricate a high resolution and high aspect ratio pattern on a surface, the negative resist used in e-beam lithography needs to have high sensitivity to electrons, small molecular size, and low thickness[44].
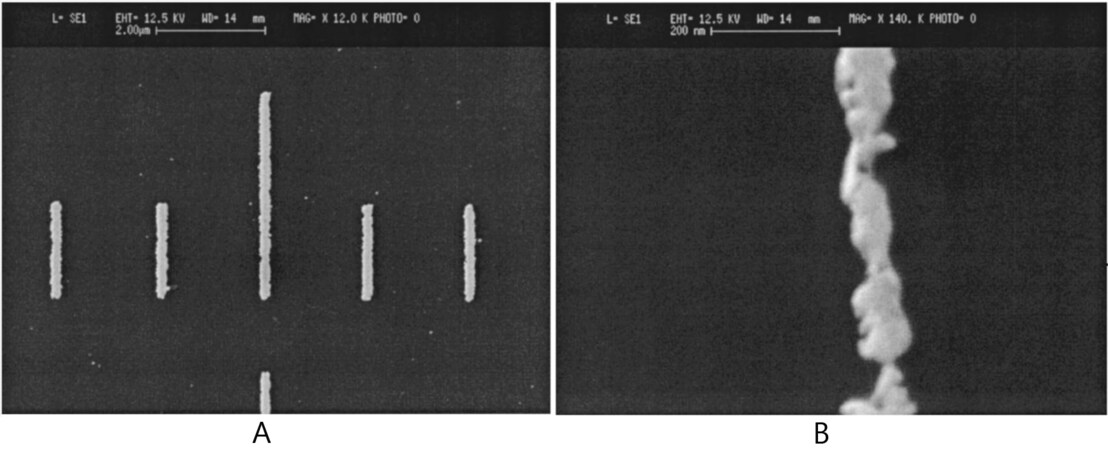
When the crosslinked BPT SAMs were first discovered by Geyer and co-workers in 1999, the authors noted that these crosslinked SAMs showed good resistance to chemical etching solution[21]. Compared to a conventional polymeric resist such as polymethylmethacrylate (PMMA), crosslinked aromatic SAMs have the potential to be used as negative resists in e-beam lithography. To test BPT SAMs for use as negative resists, Hinze and co-workers patterned BPT-coated gold substrates with an e-beam. Then, the patterned SAM-coated surface was placed into a 0.2 M KCN/1 M KOH solution for chemical etching, also called a wet etching process. After wet etching, the gold surface with crosslinked SAMs remained while the other pristine SAM areas were etched away, which showed clear patterns with a depth of 20 nm and small width of down to 10 nm [Figure 6][45].
Figure 6. SEM image of lines with a width of: (A) 100 nm, (B) 10 nm prepared using a BPT SAM negative resist. Reprinted with permission from Ref.[45], copyright 2000 AVS. BPT: Biphenyl-4-thiol; SAM: self-assembled monolayer.
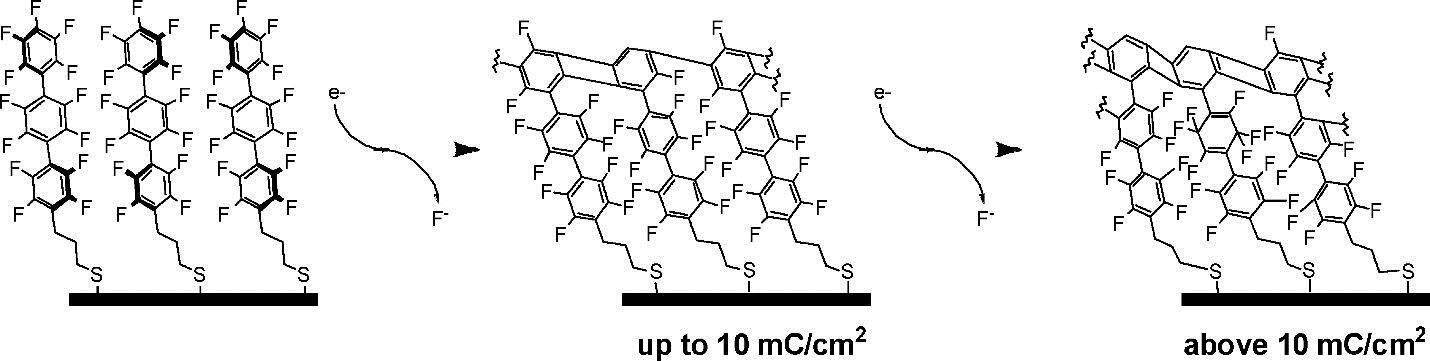
Subsequently, Yildirim and co-workers investigated the lithography properties of crosslinked aromatic SAMs containing one, two, and three phenyl rings[37]. The BPT SAM exhibits the best performance as a negative resist, as the phenyl thiol fails to form a highly crosslinked network, and the pristine terphenyl thiol SAMs are hard to remove by wet etching. Fluorinated SAMs (FSAMs) have been used for surface modification thanks to the excellent hydrophobic and low surface friction properties of fluorinated materials[46]. However, FSAMs are vulnerable to radiation damage by low-energy electrons[47]. To solve this issue, Terfort and co-workers investigated crosslinked perfluoroterphenyl-terminated SAMs exposed to low-energy electrons with a low dose (10 eV, 30 mC/cm2), as shown in Figure 7[48]. Note that the cleavage of C-F bonds observed upon irradiation follows the behavior of hydrocarbon aromatic SAMs. Additionally, the crosslinked FSAMs can be used as e-beam resists and hydrophobic coatings.
Figure 7. Schematic representation of electron-induced crosslinking of perfluoroterphenyl-terminated SAMs. Reprinted with permission from Ref.[48], copyright 2009 American Chemical Society. SAMs: Self-assembled monolayers.
In summary, BPT SAM and its derivatives have been widely used in e-beam lithography[23]. Chemical etching, direct laser patterning[49], thermodesorption[50], and SAM exchange[34,51] can be used to remove pristine SAMs on a surface to form a pattern. All the methods mentioned above utilize the high stability of crosslinked SAMs compared to pristine SAMs.
Surface functionalization
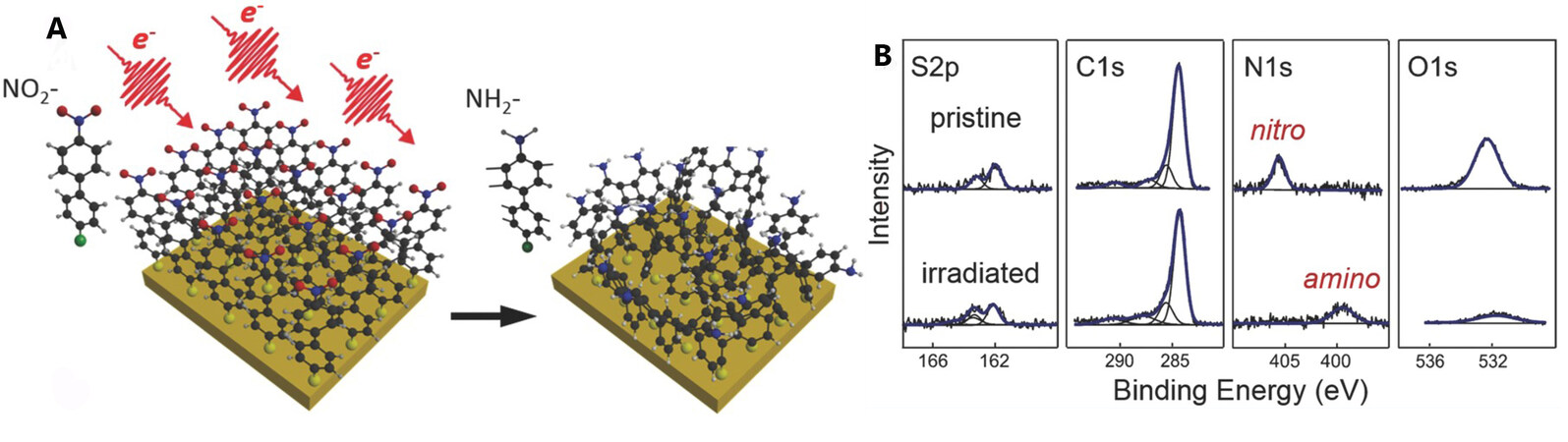
Surface functionalization plays an important role in biotechnology applications. By modifying a surface with different chemical functional groups, researchers can immobilize and detect biomolecules efficiently[52]. In 2007, Turchanin and co-workers crosslinked 4'-nitro-4-biphenylthiol (NBPT) through a stencil mask to fabricate a patterned amino-terminated SAM, as shown in Figure 8A[34,53]. XPS spectra show that after irradiation, the peak in the N 1s region shifts from 405.5 eV to 399.2 eV and the peak intensity in the O 1s region decreases significantly, which represent the full reduction of amino functional groups from nitro functional groups, as shown in Figure 8B.
Figure 8. Irradiation-induced crosslinked NBPT SAM. (A) Schematic representation of the irradiation process, (B) XPS spectra for NBPT on gold before and after irradiation. Reprinted with permission from Ref.[53], copyright 2016 Wiley. NBPT: 4'-nitro-4-biphenylthiol; SAM: self-assembled monolayer; XPS: X-ray photoelectron spectroscopy.
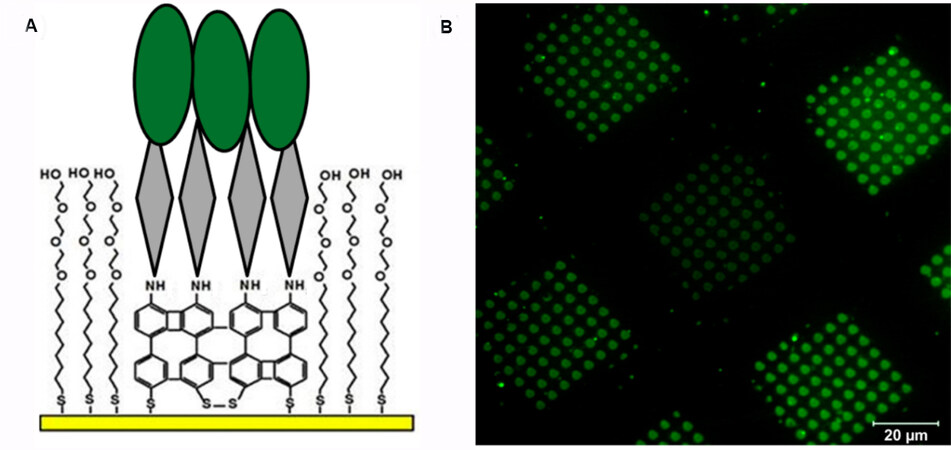
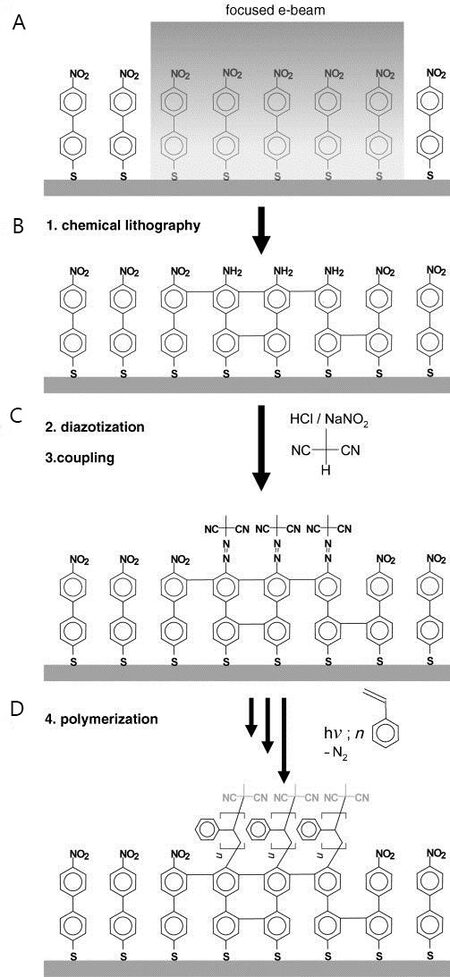
In a follow-up study, an NBPT SAM was used for the fabrication of a protein microarray for biodetection applications[54,55]. The NBPT SAM on gold was crosslinked by e-beam irradiation through a stencil mask. The remainder of the pristine NBPT SAM was exchanged with protein-resistant SAMs terminated with an ethylene glycol segment[56]. Finally, a chelator was added to the surface, followed by drop-casting of a protein solution, as shown in Figure 9A. Thanks to the strong binding between amino groups and the target protein, only the electron-irradiated areas were covered with proteins that showed strong fluorescence, as demonstrated in Figure 9B[54]. Nitrile-terminated aromatic SAMs, which also reduce to amino-terminated crosslinked SAMs upon irradiation, have been investigated by Zharnikov and co-workers[27]. Researchers found that compared to the NBPT SAM, cyanobiphenyl thiol forms less dense amino groups on the surface after irradiation due to partial defragmentation of tailgroups. For the aforementioned crosslinked perfluoroterphenyl-terminated SAMs, researchers have adopted this system for metal deposition for electronic applications, which utilize crosslinked films to prevent the intercalation of metal atoms[57].
Figure 9. (A) A schematic of the protein microarray structure. (B) Laser scanning microscopy (LSM) image of a protein microarray fabricated by a crosslinked NBPT SAM. Adapted from Ref.[54], copyright 2017 American Chemical Society. NBPT: 4'-nitro-4-biphenylthiol; SAM: self-assembled monolayer.
Surface-initiated polymerization
Surface-initiated polymerization (SIP) shows promise for protective coating applications, such as antifouling and anti-corrosion[58]. Recently, the NBPT SAM has attracted more and more attention in this application because of its precise control of polymer growth in selective areas with nanometer resolution[59,60]. In this method, NBPT SAM was patterned by e-beam lithography, resulting in a stable crosslinked amino-terminated SAM, as shown in Figure 10A and B. Next, the amino tailgroups were converted to diazo initiators in a diazotization process, as shown in Figure 10C. Finally, as shown in Figure 10D, the polymerization was started by adding styrene monomer and catalyst to the solution. After photopolymerization, electron-irradiated SAM areas yielded polystyrene (PS) brushes with a minimum thickness of 10 nm[60]. In a follow-up study, by combining a crosslinked NBPT SAM and various monomers, researchers grew homogeneous polymer brushes with thicknesses ranging from 10-300 nm precisely, which respond to pH changes and can be used for building microsensors[61].
Figure 10. Schematic representation of polymer brush fabrication. Reprinted with permission from Ref.[60], copyright 2007 Wiley.
Carbon nanomembranes (CNMS)
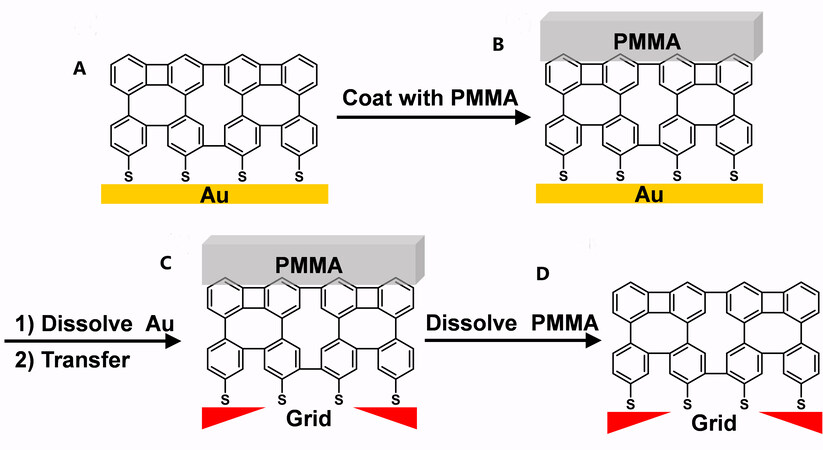
In 2005, Gölzhäuser and co-workers fabricated the first carbon nanomembranes (CNMs) from crosslinked aromatic SAMs[62]. As shown in Figure 11A and B, after the crosslinked aromatic SAMs were formed on the surface of gold, a transfer medium, PMMA, was coated on top of the SAMs. Then, the gold surface was etched as a sacrificial substrate to release the crosslinked films. Finally, CNMs were transferred onto other substrates, such as metal grids, Si, and graphene, followed by dissolution of the PMMA, as shown in Figure 11C and D.
Figure 11. Schematic representation of the transfer of CNMs onto other surfaces such as metal grids. Adapted from Ref.[23], copyright 2012 Elsevier. CNMs: Carbon nanomembranes.
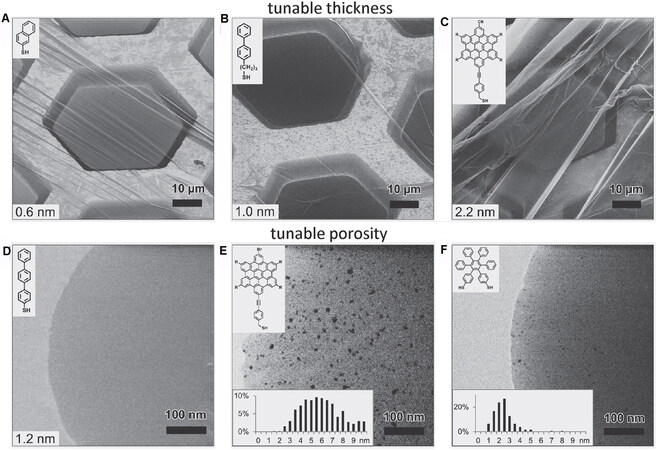
Since CNMs are converted from aromatic SAMs, their physical properties, such as thickness, rigidness, and porosity, can be tailored by using different SAM precursors[63]. Figure 12 shows helium-ion microscopy images of CNMs obtained from six different SAMs on TEM grids. From Figure 12A-C, the thickness of the pristine SAMs increases from 0.6 nm to 2.4 nm, resulting in the final thickness of the CNMs increasing accordingly. Moreover, while the use of a small SAM molecule such as that shown in Figure 12D leads to hole-free CNMs, bulkier molecules (such as the molecules in Figure 12E and F) form less-ordered SAMs and create more porous CNMs due to lower packing densities and intermolecular stacking[22]. Additionally, it is simple to pattern CNMs with uniform nanoholes as nanosieves using e-beam lithography[64].
Figure 12. HIM images of CNMs converted from various pristine SAMs and then transferred onto TEM grids. The upper left insets show the pristine SAMs. Adapted from Ref.[22], copyright 2013 American Chemical Society. HIM: Helium ion beam; CNMs: Carbon nanomembranes; SAMs: self-assembled monolayers.
Furthermore, CNMs are easily chemically functionalized by using SAM precursors with different functional groups, as used for coupling biomolecules and polymers[65]. After amino-terminated CNMs are released from gold, the thiol group on the opposite site of the CNM can couple with Au nanoparticles or other functional groups such as maleimide to form a Janus nanomembrane[66]. After years of rapid development, CNMs prepared from crosslinked aromatic SAMs have various applications in many fields, such as graphene manufacturing[67], nanofiltration[68], and microdetectors[69,70].
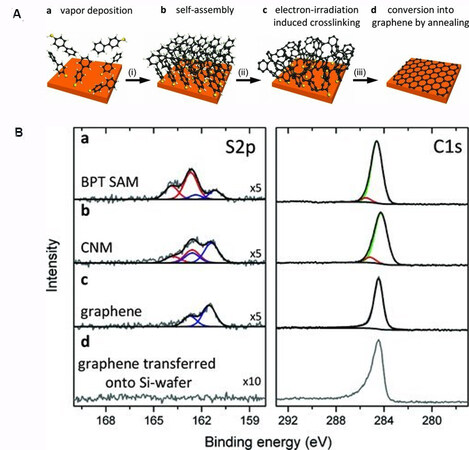
In 2013, Turchanin and co-workers grew single-layer graphene on Cu by annealing CNMs at above 800 °C for 2 h, as shown in Figure 13A[71]. The authors continued this research and fabricated patterned graphene from CNMs by using e-beam lithography[72]. From XPS spectra shown in Figure 13B, a doublet was observed at a binding energy of 161.2 eV (blue line), which was attributed to copper sulfides, and a doublet due to bound thiolate (red line) was found to completely disappear after annealing. Interestingly, the S 2p signal in the XPS spectra completely disappeared after transferring graphene to a Si-wafer, which indicated S-C bond cleavage and that sulfur atoms were left on the Cu substrate. After annealing BPT CNM into graphene, Young’s modulus was found to increase from 10 GPa to 48 GPa, indicating a significant increase in the mechanical strength[73].
Figure 13. Conversion of BPT SAMs into graphene on copper substrates: (A) Schematic representation, (B) XPS spectra for the products in each step. Adapted from Ref.[71], copyright 2013 Wiley. BPT: Biphenyl-4-thiol; SAMs: self-assembled monolayers; XPS: X-ray photoelectron spectroscopy.
For separation, thin membranes, which combine high permeability and high selectivity, are desirable in the water treatment industry[74,75]. CNMs and graphene can be fabricated into layers as thin as 1 nm, with a nanoporous structure and excellent mechanical strength, which make them excellent candidates for nanofiltration[76,77]. Furthermore, researchers have used amino-terminated CNMs to stack graphene to fabricate CNM/graphene field-effect transistors (FETs) as an alternative for graphene functionalization for biosensors and gas sensors[69,70].
CROSSLINKED OLEFINIC- AND ACETYLENIC-BASED SAMS
Beside crosslinked aromatic thiol-based SAMs, crosslinked SAMs from olefinic- and acetylenic-based alkanethiols have also been well studied by scientists. Additionally, polydiacetylene materials are a type of conducting polymer with special optical properties that have extensive applications in photonics, nanoelectronics, and biosensors[78]. When polydiacetylene materials couple with chemical and biological stimuli, their characteristic absorption peak shows a redshift. Therefore, they have a sensitive chromic response to microorganisms, viruses, and proteins, which is attractive for biosensing applications[79].
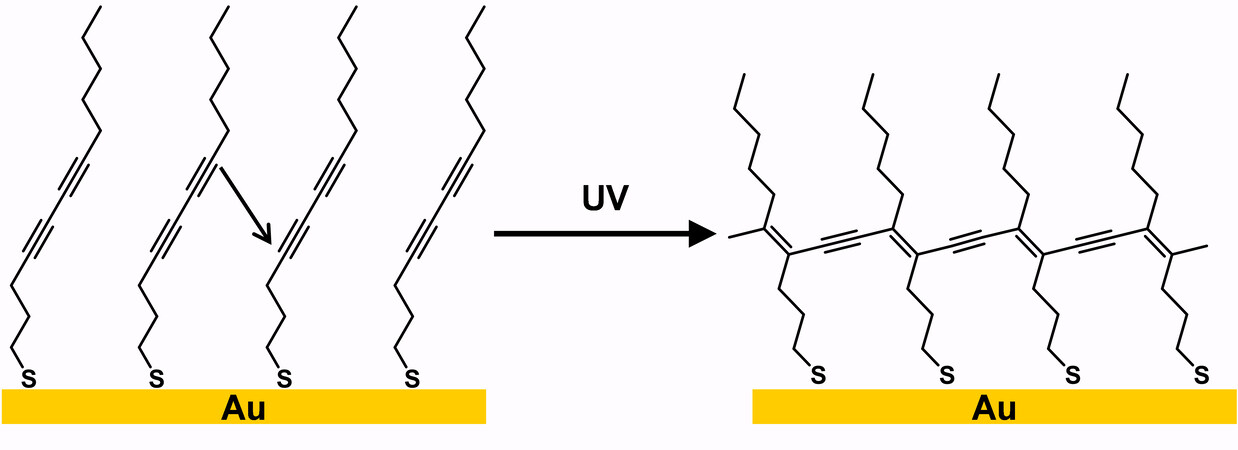
Crosslinked SAMs containing polydiacetylene were first reported by Batchelder et al.[80] in 1994. The authors deposited polymeric monolayers from diacetylene-containing disulfide onto Au (111) surfaces, as shown in Figure 14. Similar to diacetylenes in the solid-state, the chain polymerization of diacetylene thiolates (DATs) on surfaces follows some spatial conditions: the distance of the adjacent thiolates is 4.7-5.2 Å, and the distance between the C1 carbon of one diacetylene group to the C4 carbon of the adjacent diacetylene group (black arrow in Figure 14) is 3.4-4.0 Å[81,82]. In contrast, diacetylenes undergo both 1,2- and
Figure 14. Schematic representation of the polymerization process of DATs on Au. Adapted from Ref.[81], copyright 1999 American Chemical Society. DATs: Diacetylene thiolates.
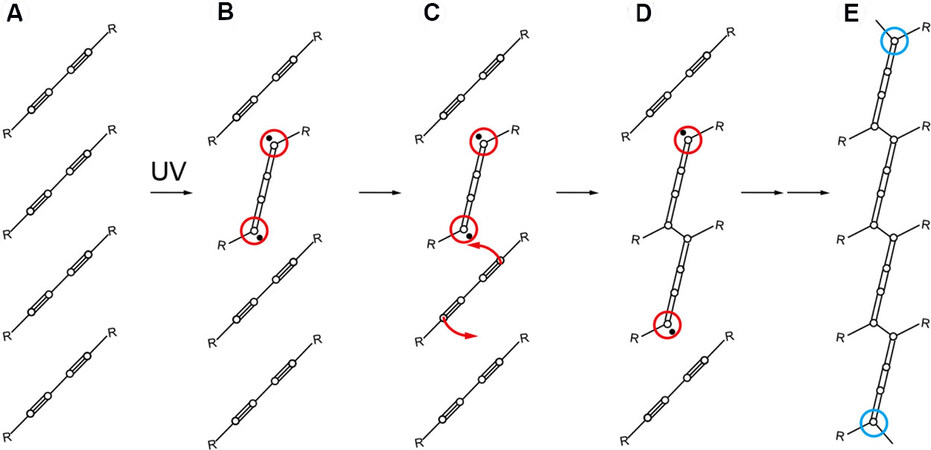
The mechanism for 1,4-photopolymerization of diacetylenes is illustrated in Figure 15[82]. First, the initiation is started by UV irradiation of DAT monomers, which generates a diradical monomer, as shown in Figure 15A and B. After that, an addition reaction occurs at both ends of the diradical monomer, as shown in Figure 15C. Finally, chain propagation at both sides [Figure 15D] is followed by the termination of reactive radicals, as suggested by the blue circles in Figure 15E. The polydiacetylene backbone contains alternating double and triple bonds, which are represented in a resonance structure of three cumulative double bonds, as shown in Figure 15E.
Figure 15. Mechanism for 1,4-photopolymerization of diacetylenes. (A) Array of diacetylene molecules. (B) An excited diradical monomer (red circles). (C) Coupling of the neighboring monomer (red arrows). (D) Diradical dimer. (E) Chain propagation reaction toward both sides until termination of reactive radicals (blue circles). Reprinted with permission from Ref.[82], copyright 2019 American Chemical Society.
In a following study, Cai et al.[81] investigated the influence of ultraviolet (UV) exposure time on the crosslinked product. They used resonance Raman spectroscopy to monitor the polydiacetylene backbone in crosslinked SAMs as a function of UV exposure time, which showed that the effective conjugation length in crosslinked DAT SAMs reached a maximum at 7 min before decreasing. This might be due to the increase in the hybridization strain in the polydiacetylene backbone as well as in the methylene chains with prolonged UV exposure. Batteas and co-workers studied the properties of crosslinked
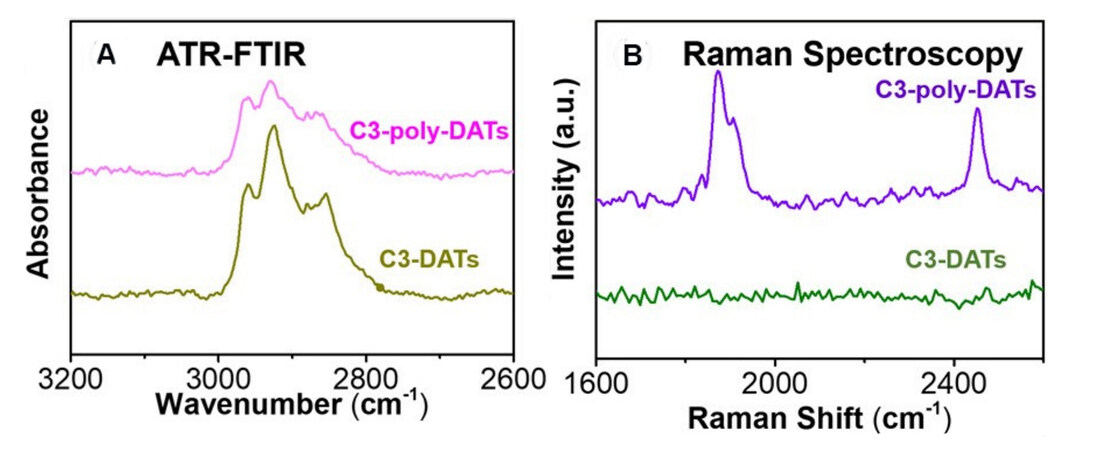
Figure 16. (A) ATR-FTIR and (B) Raman spectra for C3-DATs and C3-poly-DATs on Au (111) surfaces. Adapted from Ref.[83], copyright 2020 American Chemical Society. DATs: Diacetylene thiolates.
In a separate study, Menzel et al.[84] studied the effect of the diacetylene functional group position on crosslinked DAT SAMs. They discovered that moving the diacetylene moiety closer to the thiol headgroup increases the hybridization strain in the alkyl chain, which reduces the conversion of polymerization. In addition, with carboxylic acid-terminated DATs on Au (111), researchers were able to convert carboxylic acid into acid chloride, followed by coupling with other DATs to fabricate double and multilayer polydiacetylene coatings[85]. By using this method, scientists utilized carboxylic acid-terminated DATs as scaffolds to build polydiacetylene thin films layer-by-layer, up to a thickness of 6 layers. The authors subsequently synthesized DAT SAMs on Au (111) with terminal methyl, hydroxyl, and carboxyl acid functional groups[86]. Compared to normal alkanethiol SAMs or pristine DAT SAMs on Au surfaces, all crosslinked DAT SAMs showed excellent stability toward repeated electrochemical cycling, high temperatures of up to 200 °C, and basic solutions[86].
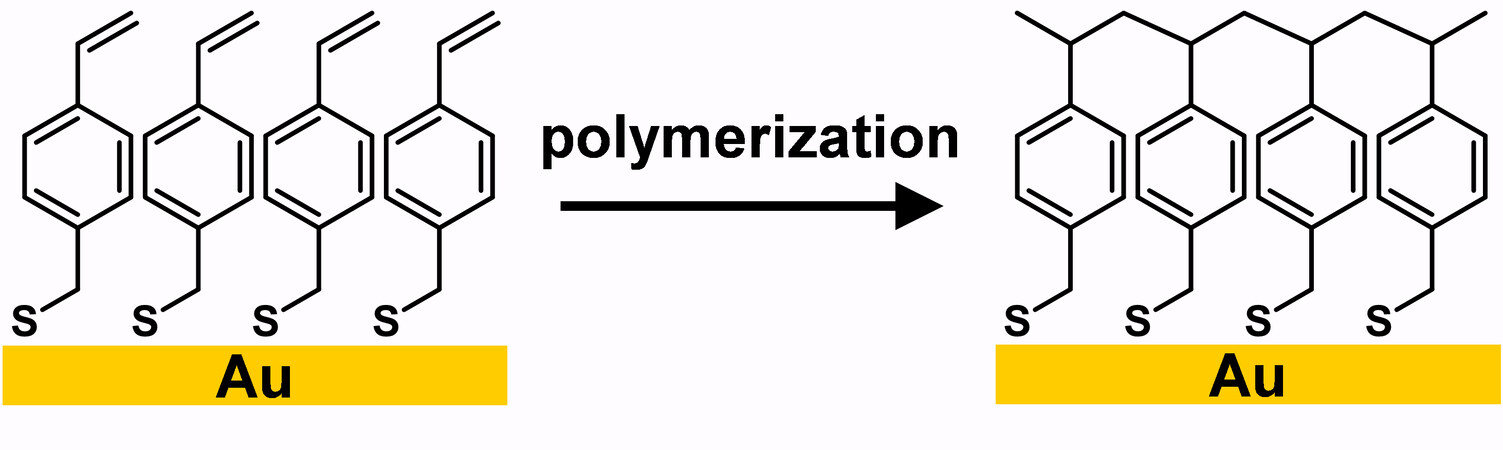
Another type of crosslinked SAM derived from olefinic-based alkanethiols is mercaptomethyl styrene, which was discovered by Schlenoff and co-workers in 1996[87]. In this case, polymerization of the absorbates was initiated by immersing the substrate in an azo initiator solution at 58 °C or by irradiating with a green laser [Figure 17]. Time-resolved SERS spectra showed a peak at 1625 cm-1 that disappeared after crosslinking, which was attributed to the double bonds of the pristine SAM.
Figure 17. Schematic representation of the polymerization of MMS on Au. Adapted from Ref.[87], copyright 1996 American Chemical Society. MMS: Mercaptomethyl styrene.
Applications
Photolithography
Crosslinked DAT SAMs have many applications in photolithography, SAM-coated nanoparticles, and single-layer polydiacetylene fabrication. Photolithography is one of the most widely used techniques in semiconductor fabrication, which transfers patterns by exposing photoresist-coated surfaces to UV through a photomask[40]. In 1995, Crooks and co-workers applied UV irradiation to a DAT SAM-coated Au (111) to fabricate patterned polydiacetylene thin films[88]. The uncrosslinked portion of the DAT SAM was stripped using electrochemical desorption, followed by KCN/KOH solution etching. STM depth profiles indicated that a clear negative pattern was formed on the Au (111), which was approximately 7-8 nm in depth. These results validated the use of DAT SAMs as ultra-thin photoresists, which crosslink under UV and protect the underneath surfaces.
Nanoparticles for antifouling
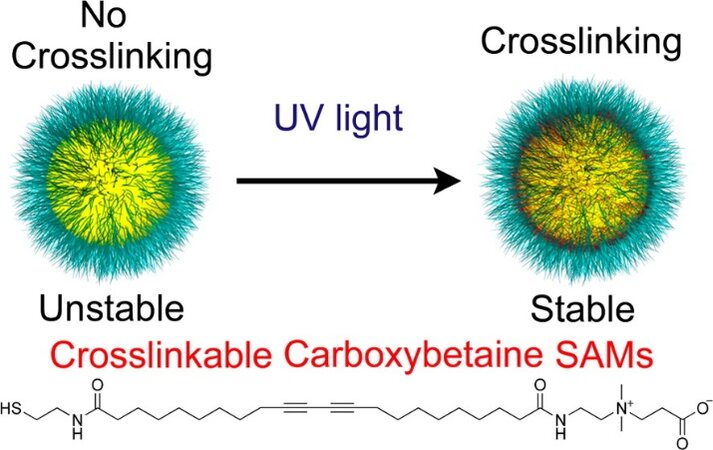
In addition to application as a negative resist in photolithography, crosslinked DAT SAMs are valuable in SAM-coated nanoparticles in biomedical applications. In 2014, Jiang and co-workers coated gold nanoparticles with SAMs containing diacetylene to test their stability in different complex media, as shown in Figure 18[89]. The carboxybetaine functional group is one of the zwitterions that are used for antifouling coatings[90]. The authors designed and synthesized carboxybetaine-terminated alkanethiol with photocrosslinkable diacetylene in the alkyl chain spacer, and then crosslinked it on gold nanoparticles using UV irradiation to enhance the antifouling property. The crosslinked SAM-coated gold nanoparticles showed high resistance to protein fouling from blood serum and decreased cell uptake. Additionally, they were stable at low/high pH and a high temperature of 80 °C.
Figure 18. A schematic showing cross-linked gold nanoparticles with CBSH-X. Reprinted with permission from Ref.[89], copyright 2014 American Chemical Society.
Single-layer polydiacetylene fabrication
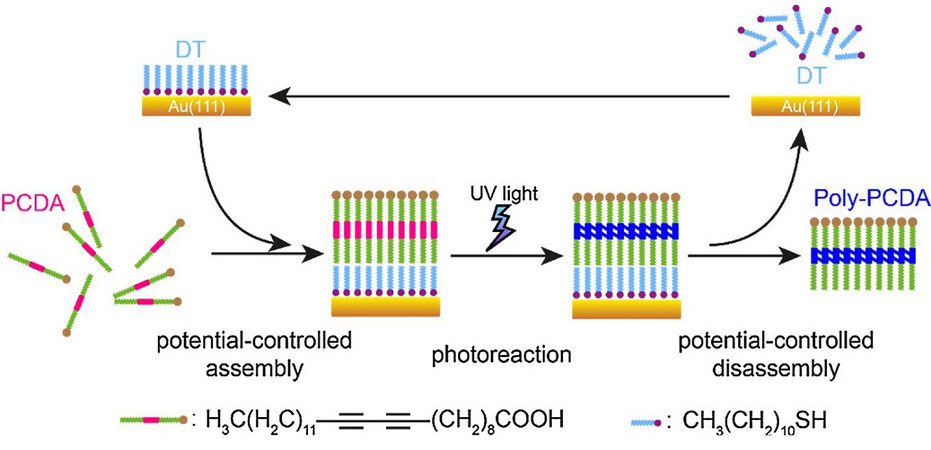
Recently, Wang et al.[91] invented a new strategy to fabricate single-layer polydiacetylene thin films using a SAM-coated gold electrode as a template. As illustrated in Figure 19, the authors deposited a functionalized gold electrode with dodecanethiol (DT), followed by the assembly of pentacosa-10,12-diynoic acid via an electrode potential, which formed a well-ordered second monolayer on the DT. After photopolymerization by UV, the polymerized monolayer of polydiacetylene was desorbed by application of an opposite electrode potential, which repeatedly worked to produce 2D polydiacetylene thin films.
Figure 19. Schematic representation of the fabrication of single-layer polydiacetylene through co-assembled PCDA and DT double layers. Reprinted with permission from Ref.[91], copyright 2017 Elsevier. PCDA: Pentacosa-10,12-diynoic acid; DT: dodecanethiol.
OTHER CROSSLINKED ALIPHATIC SAMS
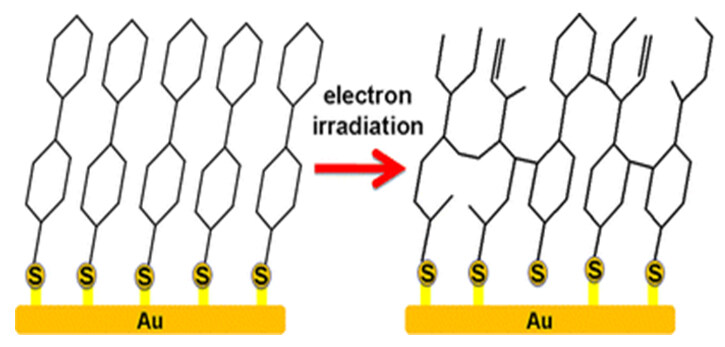
In contrast to the crosslinking of aromatic thiol-based SAMs, aliphatic thiol-based SAMs undergo decomposition and disordering upon electron irradiation[92-95]. Interestingly, in the case
Until now, the only study of irradiation-induced crosslinking of cyclic aliphatic thiol-based SAMs was reported by Zharnikov[96] and co-workers in 2012. The authors found that both SAMs generated from trans- and cis-conformations of CCHT crosslinked upon exposure to electron radiation, as shown in Figure 20. This contrasts with the linear aliphatic thiol-based SAMs, which were severely damaged when exposed to quite low irradiation doses (5-8 mC/cm2).
Figure 20. The formation of a crosslinked SAM of CCHT via electron irradiation. Reprinted with permission from Ref.[96], copyright 2012 American Chemical Society. SAM: Self-assembled monolayer; CCHT: 4-cyclohexylcyclohexanethiols.
Under electron irradiation conditions, the C-H bonds are cleaved to generate radical moieties, which are relatively stable because of the nature of the cyclic skeletons of CCHT before crosslinking to other absorbates to form a 2D molecular network. Compared to the linear aliphatic thiol SAMs, the presence of cyclic rings is the key to the dominance of crosslinking over fragmentation and desorption processes. In cases where the C-C bonds belonging to the cyclic rings are cleaved, both fragments were still attached to the molecule matrix, which then, subsequently form cross-linked bonds with neighboring molecules. Hence, the desorption of fragments is suppressed. The newly formed 2D network by crosslinking also hinders the cleavage of the headgroup-substrate S-Au bonds.
Applications
Electron-beam lithography
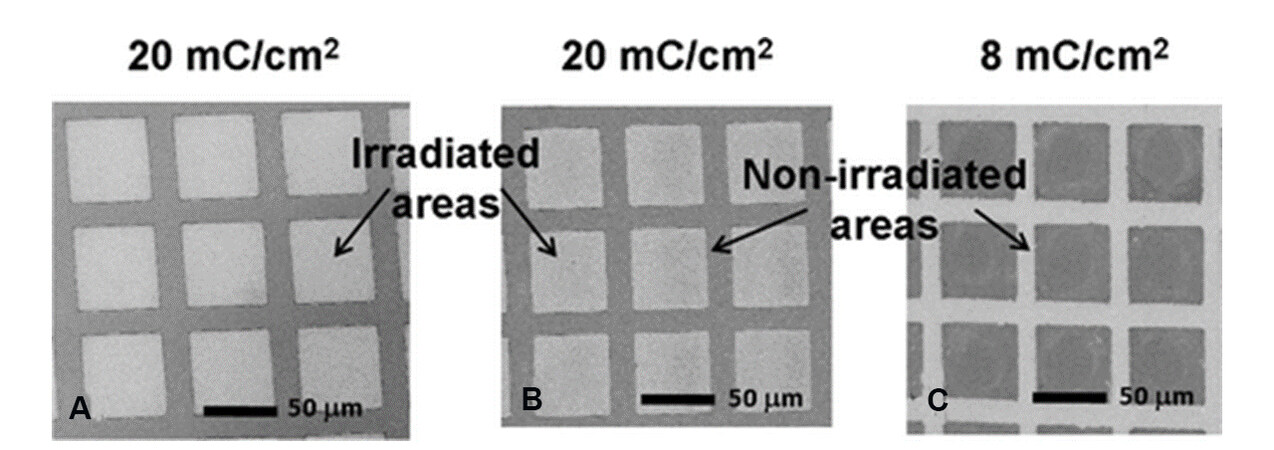
Similar to aromatic SAMs, crosslinked CCHT SAMs have been used as negative resists in e-beam lithography[96]. Figure 21 shows the SEM images of gold surfaces patterned using SAMs as
Figure 21. The SEM images of Au surfaces patterned using SAMs as resists: (A) cis-CCHT; (B) trans-CCHT, and (C) DDT. Reprinted with permission from Ref.[96], copyright 2012 American Chemical Society. CCHT: 4-cyclohexylcyclohexanethiols; DDT: dodecanethiol.
CROSSLINKED SILANE-BASED SAMS
Another important type of crosslinked SAMs are silane-based alkanethiols, which can be used in SIP[97], surface passivation[98,99], and anti-corrosion coatings[100,101]. The crosslinked silane network forms a dense film to prevent oxygen or moisture from diffusing into interfaces, which provides for long-term surface passivation. Additionally, the high thermostability of the crosslinked SAMs helps one to grow polymer brushes at high temperatures in applications involving SIP[97].
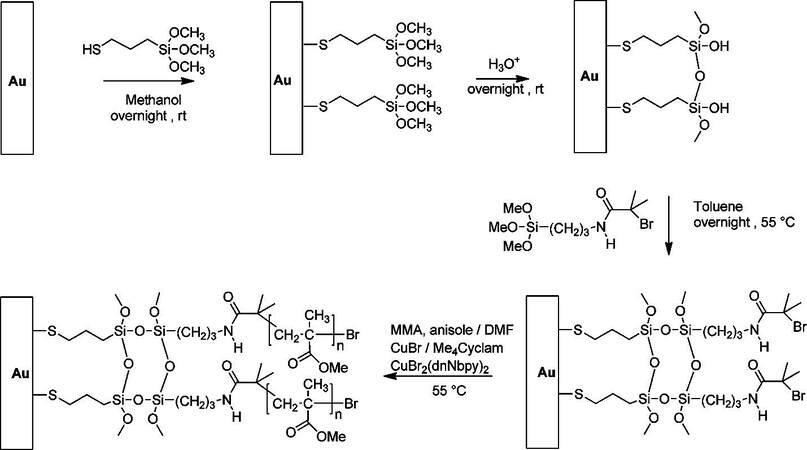
As a pristine molecule for crosslinked silane-based alkanethiols, (3-mercaptopropyl) trimethoxysilane (MPTMS) has been well studied and widely adopted in the aforementioned applications. Taking MPTMS SAM as an example, as shown in Figure 22, the formation of a crosslinked MPTMS SAM can be concluded in 3 steps: (1) MPTMS molecules are deposited onto a gold surface in anhydrous conditions to avoid self-polymerization; (2) hydrolysis of the silane converts the methoxy group (-OCH3) connected to Si to hydroxyl groups (-OH) in the presence of water; (3) at the same time, intermolecular condensation leads to the Si-O-Si structure forming a crosslinked polysiloxane network[102,103]. It should be noted that the trimethoxysilane group is less reactive to water than the trichlorosilane group, making it easier to control the polymerization of the silane on a surface[16]. In this stage, with silanol groups present on the surface, one can generate thick self-polymeric films[98] or enable grafting onto other silanes with specific functional groups[97].
Figure 22. Schematic representation of crosslinked MPTMS SAM formation on Au. Adapted from Ref.[102], copyright 2016 American Chemical Society. MPTMS: (3-mercaptopropyl) trimethoxysilane; SAM: self-assembled monolayer.
Applications
Surface-initiated polymerization
In 1994, Aramaki and co-workers coated hydroxyl-terminated alkanethiols onto a copper surface, followed by deposition of alkyltrichlorosilane[104]. The hydroxyl groups coupled with alkyl trichlorosilane resulted in a film with a crosslinked polysiloxane network. In 2011, Baker and co-workers used silane-based alkanethiols to fabricate crosslinked atom transfer radical polymerization (ATRP) initiators to grow polymer brushes on gold, as shown in Figure 23[97]. After the crosslinked MPTMS SAM was formed on Au to provide a hydroxylated surface, another silane terminated with an initiator was grafted onto the MPTMS SAM, which formed a second layer of crosslinked interchain polysiloxane. After adding methyl methacrylate monomer and catalyst, the system was heated up from 50 °C to 100 °C to initiate polymerization of PMMA from Au surfaces.
Figure 23. Surface-initiated polymerization of PMMA from crosslinked ATRP initiators on Au. Reprinted with permission from Ref.[97], copyright 2011 American Chemical Society. PMMA: Polymethylmethacrylate; ATRP: atom transfer radical polymerization.
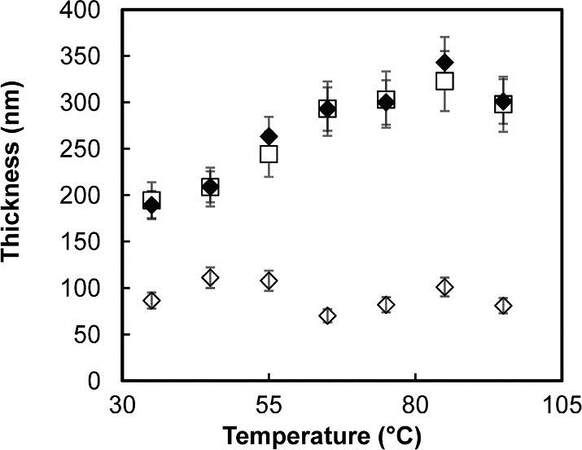
The authors found that normal alkanethiols with ATRP initiator on gold led to the growth of thinner polymers compared to silane SAMs with ATRP initiator on a SiO2 substrate due to the poor thermostability of uncrosslinked initiators. In contrast to a crosslinked polysiloxane primer layer, polymer brushes grown from ATRP initiators on Au reached the same thickness as on a SiO2 substrate, as shown in Figure 24. Thanks to the highly stable Si-O bond of crosslinked silane-based SAMs, the novel ATRP initiator allowed SIP at higher temperatures of up to 100 °C, which provides polymer growth from less reactive monomers such as styrene and vinyl pyridine.
Figure 24. Temperature-dependent surface-initiated polymerization of MMA from various initiators; ◊ PMMA grown from a standard initiator; ⧫ PMMA grown from the crosslinked initiator; and ◻ PMMA grown from a silane initiator on SiO2. Reprinted with permission from Ref.[97], copyright 2011 American Chemical Society. PMMA: Polymethylmethacrylate; MMA: methyl methacrylate.
Compared to the aromatic thiol-based SAMs, homogeneous SIP from crosslinked silane-based alkanethiols is cost-efficient because it does not need an expensive e-beam lithography system for the crosslinking process. However, it is difficult to pattern silane-based SAMs since it has been reported that silanes are not compatible with microcontact printing techniques[16].
Surface passivation
GaAs is a promising III-V semiconductor material that is widely used in devices such as photovoltaics, light-emitting diodes, photonics, and radio frequency transistors, thanks to its high electron mobility and direct bandgap[105,106]. To date, GaAs solar cells have shown record-breaking conversion efficiencies, ~29% for single-junction cells[107]. However, GaAs can become oxidized in the ambient environment, and the oxide layer formed on GaAs can significantly decrease the solar cell efficiency[108]. Hence, surface passivation of GaAs devices to prevent oxidation and corrosion is the key to enhancing the durability and performance of GaAs devices. Because sulfur species can bind to both Ga and As atoms to passivate unsaturated binding sites and form a thin protective layer on a GaAs surface, scientists have attempted to use several sulfur compounds to passivate a GaAs surface, such as (NH4)2S, Na2S, and normal alkanethiols[109,110]. However, when GaAs is treated with the first two inorganic salts, toxic H2S is produced. Moreover, passivation layers on the GaAs surface formed by all three kinds of compounds are less than 2 nm thick, which means that oxygen can still diffuse into the protective film to oxidize the GaAs surface after 2 days[111].
In 1997, Kauffman and co-workers discovered that MPTMS SAM was a good candidate for GaAs surface passivation[99]. After years of development, MPTMS has been widely used in GaAs devices such as solar cells and GaAs-based sensors, and the process used for surface passivation has become efficient and mature[98]. As shown in Figure 25A-C, after hydrolysis in solutions of concentrated MPTMS and NH4OH, the MPTMS monolayer formed on GaAs is crosslinked with other MPTMS molecules in the solution phase, which forms a 3D polysiloxane network. Finally, a relatively thick polymeric layer on the GaAs surface is generated, with the thickness of the resulting films ranging from 15 nm to 33 nm, which is controlled by varying the concentration of the MPTMS and NH4OH solutions[98]. The results show that GaAs devices with MPTMS coatings show long-term stability in air or aqueous environments for at least 10 days without sacrificing their original performance.
Figure 25. Schematic representation of an MPTMS coating on the surface of GaAs: (A) cleaned and etched GaAs sample, (B) primary layer formation, and (C) polymer formation. Reprinted with permission from Ref.[102], copyright 2016 American Chemical Society. MPTMS: (3-mercaptopropyl) trimethoxysilane.
Moreover, in 2002, Seitz and co-workers achieved polymerization of MPTMS in a solution sol-gel process for GaAs surface passivation[111]. In the follow-up research, this process was further developed to be used as an anti-corrosion coating for copper by Sui et al.[101]. Unlike the method introduced above, a polysiloxane network was formed by the polymerization of MPTMS in solution before being coated onto the copper surface.
CROSSLINKED BORONIC ACID-BASED SAMS
In 1994, Whiteside and co-workers utilized boronic acid-based alkanethiols to fabricate a crosslinked boronic anhydride surface[112]. Although this research was focused on boronic acid-based SAMs[113], subsequent research for boronic acid-based SAMs for crosslinking is limited. Additionally, there exists only limited research about the application of this kind of crosslinked SAM system.
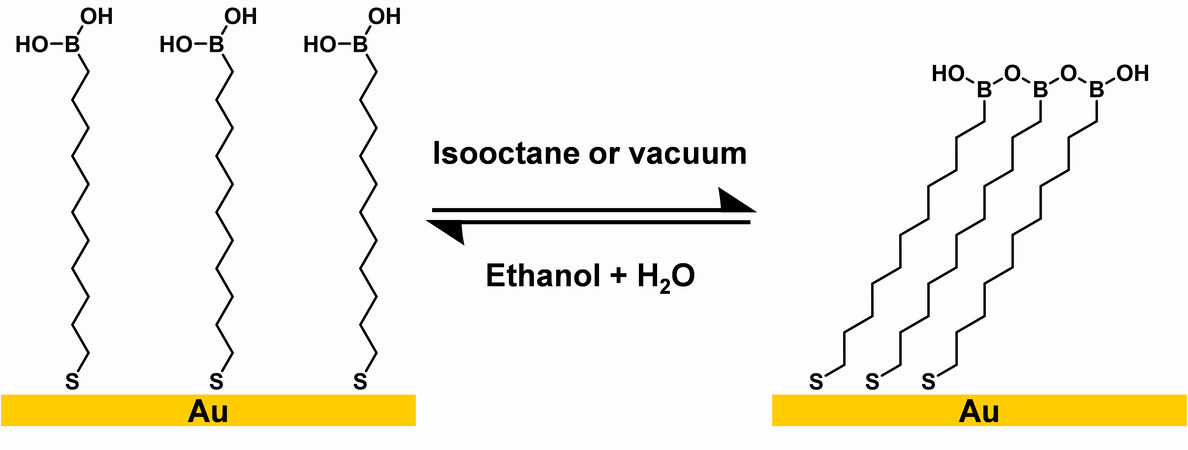
A crosslinked boronic anhydride surface was first fabricated by the condensation of 11-mercaptoundecanyl-l-boronic acid, HS(CH2)nB(OH)2 in dry hydrocarbon solvents, such as isooctane, or under vacuum, as shown in Figure 26[112]. This reaction is reversible, and the crosslinked film can be converted back into a pristine monolayer in aqueous ethanol. The author also found that in dry hydrocarbon solvents, HS(CH2)nB(OH)2 molecules in the solution phase reversibly turn into trimers that adsorb onto gold and form a hydrophobic boroxine bilayer. The resulting hydrophobic bilayer can be converted into a hydrophilic monolayer again in aqueous ethanol. Similar to hydroxyl-terminated SAMs, both the HS(CH2)nB(OH)2 SAM and its crosslinked film can couple with alkyltrichlorosilanes irreversibly, turning into a borosilicate network.
Applications
Thermally stable coatings
The thermal desorption results show that the crosslinked boronic anhydride SAM is five times more stable compared to the structurally analogous 11-hydroxyundecane-l-thiol at 147 °C. However, it is difficult to tune the thickness or crosslinked area of crosslinked boronic acid-based SAMs. Moreover, the formation of crosslinked boronic anhydride surface is reversible. These drawbacks hinder the application of crosslinked boronic acid-based SAMs compared to other crosslinked systems such as aromatic thiol-based SAMs.
Figure 26. Schematic representation of a crosslinked boronic anhydride surface. Adapted from Ref.[112], copyright 1994 American Chemical Society.
CROSSLINKED SAMS REALIZED BY HYDROGEN BONDING
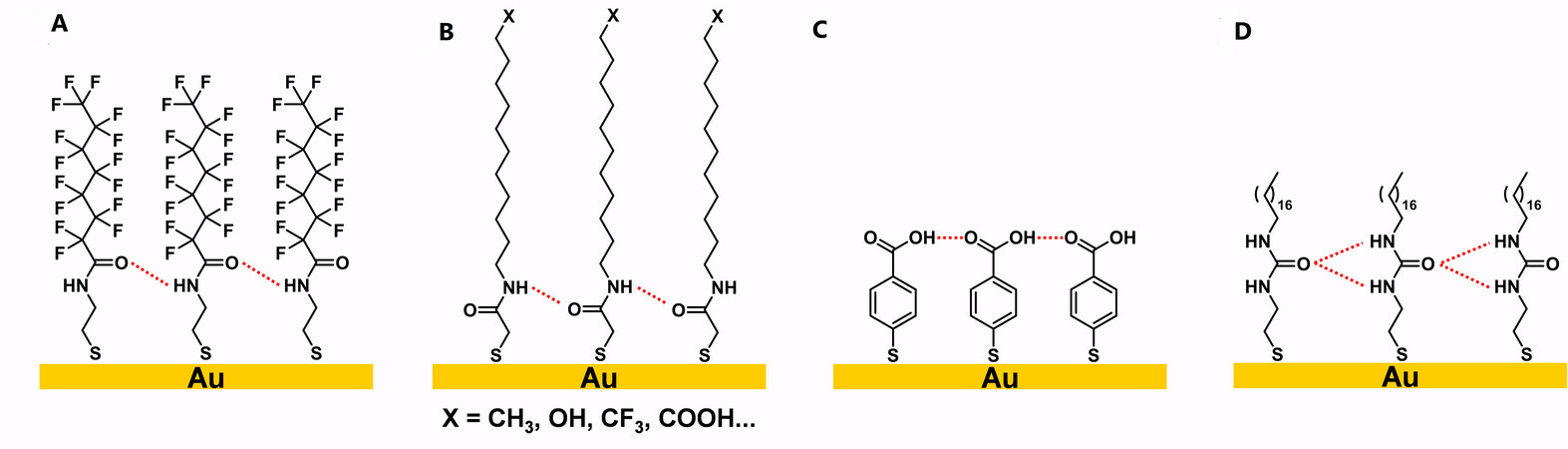
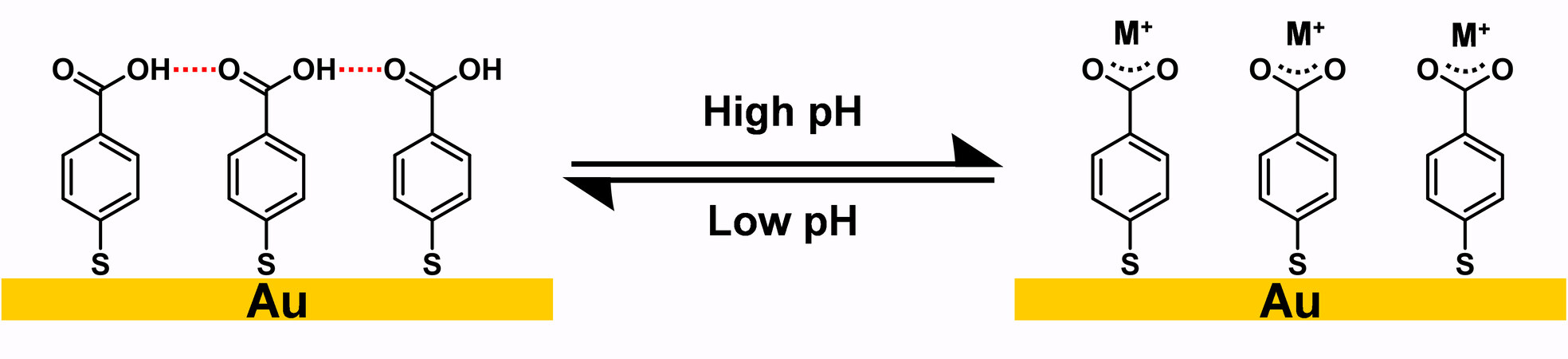
In addition to crosslinking with covalent bonding, another important type of crosslinked SAMs are SAMs with noncovalent interaction such as hydrogen bonding. Generally, the hydrogen bonding within these crosslinked SAMs arises from the amide functional group or carboxylic acid group in the tailgroups. In 1994, Rabolt and co-workers synthesized semifluorinated amidethiol, which was the first reported crosslinked SAM with hydrogen bonding, as shown in Figure 27A[114]. In a follow-up study, Whitesides and co-workers continued this research and synthesized a series of amidethiol SAMs, which were terminated with different functional groups, such as CH3, OH, CF3, COOH, etc., as shown in Figure 27B[115]. In 1997, Sastry et al.[116] prepared a 4-carboxythiophenol SAM, with the carboxylic acid moieties forming a hydrogen-bonding network at low pH, as shown in Figure 27C. In 2005, Kim et al.[117] synthesized SAMs with a urea moiety on gold, which formed a hydrogen-bonding network, as shown in Figure 27D.
When SAMs containing carboxylic acid, amide, and urea groups are absorbed on gold, hydrogen-bonding networks are formed spontaneously. In the case of the SAM containing a urea moiety in Figure 27D, infrared reflection-absorption (IRRA) spectra obtained for the SAM on gold show almost no amide I band, which appears at 1621 cm-1 in the spectra obtained for the bulk molecules[117]. This result proved that hydrogen bonding exists in the SAM network, which is composed of N-H and C=O bonds parallel to the surface, since only vibrations aligned with the surface normal appear in the IRRA spectra due to the metal-surface selection rule. In 2016, Thomas et al.[118] used STM to map the hydrogen-bonding networks formed in SAMs with an amide group. The submolecular-resolution STM images showed a linear pattern of molecule orientation (tilts) affected by hydrogen bonding, which further confirmed the existence of hydrogen bonding in the SAM network.
Applications
Selective binding of cations on surfaces
In the follow-up research, Liedberg and co-workers studied the thermal stability of SAMs influenced by hydrogen bonding, as measured by temperature-programmed desorption (TPD)[119]. They noted that SAMs with amide moieties show better thermostability compared to their normal alkanethiol analogs, with a temperature difference of 25 K for the thermodesorption, due to the hydrogen bonding. Moreover,
Figure 28. Schematic representation of 4-carboxythiophenol SAM on Au at low pH, and ion adsorption [M+ = Cd(OH)+ or Pb(OH)+] at high pH. Adapted from Ref.[116], copyright 1997 American Chemical Society. SAM: Self-assembled monolayer.
CONCLUSION AND OUTLOOK
In this review, we highlighted a variety of crosslinked SAMs: (1) aromatic thiol-based SAMs; (2) olefinic- and acetylenic-based SAMs; (3) other aliphatic SAMs; (4) silane-based SAMs; (5) boronic acid-based SAMs; and (6) SAMs realized by hydrogen bonding. Their methods of formation including the conditions for crosslinking, the structures of the crosslinked products, and their interfacial properties were discussed. Furthermore, we highlighted the applications of such crosslinked SAMs in materials science, such as the fabrication of carbon nanomembranes, lithography, protective coatings, surface functionalization, and surface-initiated polymerization.
The rapid development in semiconductor industry has attracted increasing attention from scientists and engineers seeking to develop new methods to fabricate nanostructures for next-generation semiconductor chips. Although it remains difficult to achieve uniform deposition of SAMs at a 300-mm-wafer scale, and stability still limits the application of uncrosslinked SAMs under etching or epitaxy conditions, we believe that crosslinked SAMs are attractive candidates in future semiconductor manufacturing industries. Importantly, with less than 5-nanometer thicknesses, crosslinked SAMs can tolerate extreme UV photolithography and even X-ray lithography under nanofabrication conditions. We hope this review offers insight into the structure-property relationships of crosslinked SAMs, as well as inspires researchers toward the development of new types of SAMs with enhanced stabilities.
DECLARATIONS
Authors’ contributionPrepared the original draft: Yu T, Marquez MD, Tran HV
Edited the draft and added additional written content: Lee TR
Availability of data and materialsNot applicable.
Financial support and sponsorshipWe thank the National Science Foundation (CHE-2109174), the Robert A Welch Foundation (Grant No. E-1320), and the Texas Center for Superconductivity at the University of Houston for the support.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Nuzzo RG, Allara DL. Adsorption of bifunctional organic disulfides on gold surfaces. J Am Chem Soc 1983;105:4481-3.
2. Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc 1989;111:321-35.
3. Nuzzo RG, Zegarski BR, Dubois LH. Fundamental studies of the chemisorption of organosulfur compounds on gold(111). Implications for molecular self-assembly on gold surfaces. J Am Chem Soc 1987;109:733-40.
7. Wilbur JL, Kumar A, Kim E, Whitesides GM. Microfabrication by microcontact printing of self-assembled monolayers. Adv Mater 1994;6:600-4.
8. Doms M, Feindt H, Kuipers WJ, et al. Hydrophobic coatings for MEMS applications. J Micromech Microeng 2008;18:055030.
9. Yu T, Marquez MD, Zenasni O, Lee TR. Mimicking polymer surfaces using cyclohexyl- and perfluorocyclohexyl-terminated self-assembled monolayers. ACS Appl Nano Mater 2019;2:5809-16.
10. Maboudian R, Ashurst W, Carraro C. Self-assembled monolayers as anti-stiction coatings for MEMS: characteristics and recent developments. Sensors and Actuators A: Physical 2000;82:219-23.
11. Kasai T, Bhushan B, Kulik G, Barbieri L, Hoffmann P. Micro∕nanotribological study of perfluorosilane SAMs for antistiction and low wear. J Vac Sci Technol B 2005;23:995.
12. Bhushan B, Kasai T, Kulik G, Barbieri L, Hoffmann P. AFM study of perfluoroalkylsilane and alkylsilane self-assembled monolayers for anti-stiction in MEMS/NEMS. Ultramicroscopy 2005;105:176-88.
13. Kim S, Yoo H. Self-assembled monolayers: versatile uses in electronic devices from gate dielectrics, dopants, and biosensing linkers. Micromachines (Basel) 2021;12:565.
14. Grönbeck H, Curioni A, Andreoni W. Thiols and disulfides on the Au(111) surface: the headgroup - gold interaction. J Am Chem Soc 2000;122:3839-42.
15. Crudden CM, Horton JH, Ebralidze II, et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat Chem 2014;6:409-14.
16. Onclin S, Ravoo BJ, Reinhoudt DN. Engineering silicon oxide surfaces using self-assembled monolayers. Angew Chem Int Ed Engl 2005;44:6282-304.
17. Delamarche E, Michel B, Kang H, Gerber C. Thermal stability of self-assembled monolayers. Langmuir 1994;10:4103-8.
19. Allara DL, Parikh AN, Rondelez F. Evidence for a unique chain organization in long chain silane monolayers deposited on two widely different solid substrates. Langmuir 1995;11:2357-60.
20. Srisombat L, Jamison AC, Lee TR. Stability: a key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2011;390:1-19.
21. Geyer W, Stadler V, Eck W, Zharnikov M, Gölzhäuser A, Grunze M. Electron-induced crosslinking of aromatic self-assembled monolayers: negative resists for nanolithography. Appl Phys Lett 1999;75:2401-3.
22. Angelova P, Vieker H, Weber NE, et al. A universal scheme to convert aromatic molecular monolayers into functional carbon nanomembranes. ACS Nano 2013;7:6489-97.
23. Turchanin A, Gölzhäuser A. Carbon nanomembranes from self-assembled monolayers: Functional surfaces without bulk. Progress in Surface Science 2012;87:108-62.
24. Schmid M, Wan X, Asyuda A, Zharnikov M. Modification of self-assembled monolayers by electron irradiation: the effect of primary energy (10-500 eV). J Phys Chem C 2019;123:28301-9.
25. Tong Y, Berdiyorov GR, Sinopoli A, Madjet ME, Esaulov VA, Hamoudi H. An estimation on the mechanical stabilities of SAMs by low energy Ar+ cluster ion collision. Sci Rep 2021;11:12772.
26. Eck W, Stadler V, Geyer W, Zharnikov M, Gölzhäuser A, Grunze M. Generation of surface amino groups on aromatic self-assembled monolayers by low energy electron beams - a first step towards chemical lithography. Adv Mater 2000;12:805-8.
27. Meyerbröker N, Zharnikov M. Modification of nitrile-terminated biphenylthiol self-assembled monolayers by electron irradiation and related applications. Langmuir 2012;28:9583-92.
28. Turchanin A, Käfer D, El-Desawy M, Wöll C, Witte G, Gölzhäuser A. Molecular mechanisms of electron-induced cross-linking in aromatic SAMs. Langmuir 2009;25:7342-52.
29. Houplin J, Dablemont C, Sala L, Lafosse A, Amiaud L. Electron processing at 50 eV of terphenylthiol self-assembled monolayers: contributions of primary and secondary electrons. Langmuir 2015;31:13528-34.
30. Amiaud L, Houplin J, Bourdier M, et al. Low-energy electron induced resonant loss of aromaticity: consequences on cross-linking in terphenylthiol SAMs. Phys Chem Chem Phys 2014;16:1050-9.
32. Zharnikov M, Grunze M. Modification of thiol-derived self-assembling monolayers by electron and x-ray irradiation: scientific and lithographic aspects. J Vac Sci Technol B 2002;20:1793.
33. Zhang X, Vieker H, Beyer A, Gölzhäuser A. Fabrication of carbon nanomembranes by helium ion beam lithography. Beilstein J Nanotechnol 2014;5:188-94.
34. Turchanin A, Schnietz M, El-Desawy M, Solak HH, David C, Gölzhäuser A. Fabrication of molecular nanotemplates in self-assembled monolayers by extreme-ultraviolet-induced chemical lithography. Small 2007;3:2114-9.
35. Cabrera-Sanfelix P, Arnau A, Sánchez-Portal D. First-principles investigation of electron-induced cross-linking of aromatic self-assembled monolayers on Au(111). Phys Chem Chem Phys 2010;12:1578-84.
36. Koch S, Kaiser CD, Penner P, et al. Amplified cross-linking efficiency of self-assembled monolayers through targeted dissociative electron attachment for the production of carbon nanomembranes. Beilstein J Nanotechnol 2017;8:2562-71.
37. Yildirim C, Füser M, Terfort A, Zharnikov M. Modification of aromatic self-assembled monolayers by electron irradiation: basic processes and related applications. J Phys Chem C 2017;121:567-76.
38. Mrugalla A, Schnack J. Classical molecular dynamics investigations of biphenyl-based carbon nanomembranes. Beilstein J Nanotechnol 2014;5:865-71.
39. Ishida T, Hara M, Kojima I, et al. High resolution X-ray photoelectron spectroscopy measurements of octadecanethiol self-assembled monolayers on Au(111). Langmuir 1998;14:2092-6.
40. Smith BW. Optical projection lithography. In Feldman M. Ed. Nanolithography. New York: Woodhead Publishing; 2014. p. 1-41.
42. Groves TR. Electron beam lithography. In: Feldman M. Ed. Nanolithography. New York: Woodhead Publishing; 2014. P. 80-115.
43. Tseng A, Kuan Chen, Chen C, Ma K. Electron beam lithography in nanoscale fabrication: recent development. IEEE Trans Electron Packag Manufact 2003;26:141-9.
44. Gölzhäuser A, Eck W, Geyer W, et al. Chemical nanolithography with electron beams. Adv Mater ;13:803-6.
45. Gölzhäuser A, Geyer W, Stadler V, et al. Nanoscale patterning of self-assembled monolayers with electrons. J Vac Sci Technol B 2000;18:3414.
46. Barriet D. Fluorinated self-assembled monolayers: composition, structure and interfacial properties. Current Opinion in Colloid & Interface Science 2003;8:236-42.
47. Frey S, Heister K, Zharnikov M, Grunze M. Modification of semifluorinated alkanethiolate monolayers by low energy electron irradiation. Phys Chem Chem Phys ;2:1979-87.
48. Chesneau F, Hamoudi H, Schüpbach B, Terfort A, Zharnikov M. Modification of self-assembled monolayers of perfluoroterphenyl-substituted alkanethiols by low-energy electrons. J Phys Chem C 2011;115:4773-82.
49. Frese N, Scherr J, Beyer A, et al. Multicomponent patterned ultrathin carbon nanomembranes by laser ablation. Applied Surface Science 2018;427:126-30.
50. Turchanin A, El-desawy M, Gölzhäuser A. High thermal stability of cross-linked aromatic self-assembled monolayers: nanopatterning via selective thermal desorption. Appl Phys Lett 2007;90:053102.
51. Beyer A, Godt A, Amin I, et al. Fully cross-linked and chemically patterned self-assembled monolayers. Phys Chem Chem Phys 2008;10:7233-8.
52. Tran KT, Nguyen TD. Lithography-based methods to manufacture biomaterials at small scales. Journal of Science: Advanced Materials and Devices 2017;2:1-14.
54. Kankate L, Aguf A, Großmann H, et al. Vapor phase exchange of self-assembled monolayers for engineering of biofunctional surfaces. Langmuir 2017;33:3847-54.
55. Turchanin A, Tinazli A, El-desawy M, et al. Molecular self-assembly, chemical lithography, and biochemical tweezers: a path for the fabrication of functional nanometer-scale protein arrays. Adv Mater 2008;20:471-7.
56. Meyerbröker N, Li Z, Eck W, Zharnikov M. Biocompatible nanomembranes based on pegylation of cross-linked self-assembled monolayers. Chem Mater 2012;24:2965-72.
57. Chesneau F, Terfort A, Zharnikov M. Nickel deposition on fluorinated, aromatic self-assembled monolayers: chemically induced cross-linking as a tool for the preparation of well-defined top metal films. J Phys Chem C 2014;118:11763-73.
58. Kuang J, Messersmith PB. Universal surface-initiated polymerization of antifouling zwitterionic brushes using a mussel-mimetic peptide initiator. Langmuir 2012;28:7258-66.
59. Schmelmer U, Jordan R, Geyer W, et al. Surface-initiated polymerization on self-assembled monolayers: amplification of patterns on the micrometer and nanometer scale. Angew Chem Int Ed Engl 2003;42:559-63.
62. Eck W, Küller A, Grunze M, Völkel B, Gölzhäuser A. Freestanding nanosheets from crosslinked biphenyl self-assembled monolayers. Adv Mater 2005;17:2583-7.
64. Schnietz M, Turchanin A, Nottbohm CT, et al. Chemically functionalized carbon nanosieves with 1-nm thickness. Small 2009;5:2651-5.
65. Kruk M, Neumann C, Frey M, Kozieł K, Turchanin A, Cyganik P. Odd-even effect in electron beam irradiation of hybrid aromatic-aliphatic self-assembled monolayers of fatty acid. J Phys Chem C 2021;125:9310-8.
66. Zheng Z, Zhang X, Neumann C, et al. Hybrid van der Waals heterostructures of zero-dimensional and two-dimensional materials. Nanoscale 2015;7:13393-7.
67. Turchanin A. Graphene growth by conversion of aromatic self-assembled monolayers. ANNALEN DER PHYSIK 2017;529:1700168.
68. Yang Y, Dementyev P, Biere N, et al. Rapid water permeation through carbon nanomembranes with sub-nanometer channels. ACS Nano 2018;12:4695-701.
69. Zhang X, Marschewski E, Penner P, et al. Large-area all-carbon nanocapacitors from graphene and carbon nanomembranes. ACS Nano 2018;12:10301-9.
70. Woszczyna M, Winter A, Grothe M, et al. All-carbon vertical van der Waals heterostructures: non-destructive functionalization of graphene for electronic applications. Adv Mater 2014;26:4831-7.
71. Matei DG, Weber NE, Kurasch S, et al. Functional single-layer graphene sheets from aromatic monolayers. Adv Mater 2013;25:4146-51.
72. Weber NE, Wundrack S, Stosch R, Turchanin A. Direct growth of patterned graphene. Small 2016;12:1440-5.
73. Turchanin A, Beyer A, Nottbohm CT, et al. One nanometer thin carbon nanosheets with tunable conductivity and stiffness. Adv Mater 2009;21:1233-7.
75. Araújo LCA, Costa LP. Functionalized carbon nano-membranes based devices for water purification technology. In: Environmental Applications of Carbon Nanomaterials-Based Devices. 2021. p. 331-46.
76. Dementyev P, Wilke T, Naberezhnyi D, Emmrich D, Gölzhäuser A. Vapour permeation measurements with free-standing nanomembranes. Phys Chem Chem Phys 2019;21:15471-7.
77. Yang Y, Hillmann R, Qi Y, et al. Ultrahigh ionic exclusion through carbon nanomembranes. Adv Mater 2020;32:e1907850.
78. Weston M, Tjandra AD, Chandrawati R. Tuning chromatic response, sensitivity, and specificity of polydiacetylene-based sensors. Polym Chem 2020;11:166-83.
79. Reppy MA, Pindzola BA. Biosensing with polydiacetylene materials: structures, optical properties and applications. Chem Commun (Camb) 2007;42:4317-38.
80. Batchelder DN, Evans SD, Freeman TL, Haeussling L, Ringsdorf H, Wolf H. Self-assembled monolayers containing polydiacetylenes. J Am Chem Soc 1994;116:1050-3.
81. Cai M, Mowery MD, Menzel H, Evans CE. Fabrication of extended conjugation length polymers within diacetylene monolayers on au surfaces: influence of UV exposure time. Langmuir 1999;15:1215-22.
82. Takajo D, Sudoh K. Mechanism of chain polymerization in self-assembled monolayers of diacetylene on the graphite surface. Langmuir 2019;35:2123-8.
83. Wu F, Bhupathiraju NVSDK, Brown A, Liu Z, Drain CM, Batteas JD. Mechanical and electronic properties of diacetylene and polydiacetylene self-assembled monolayers on Au(111). J Phys Chem C 2020;124:4081-9.
84. Menzel H, Mowery MD, Cai M, Evans CE. Vertical positioning of internal molecular scaffolding within a single molecular layer. J Phys Chem B 1998;102:9550-6.
85. Kim T, Crooks RM, Tsen M, Sun L. Polymeric self-assembled monolayers. 2. Synthesis and characterization of self-assembled polydiacetylene mono- and multilayers. J Am Chem Soc 1995;117:3963-7.
86. Kim T, Chan KC, Crooks RM. Polymeric self-assembled monolayers. 4. chemical, electrochemical, and thermal stability of ω-functionalized, self-assembled diacetylenic and polydiacetylenic monolayers. J Am Chem Soc 1997;119:189-93.
87. Ford JF, Vickers TJ, Mann CK, Schlenoff JB. Polymerization of a thiol-bound styrene monolayer. Langmuir 1996;12:1944-6.
88. Chan KC, Kim T, Schoer JK, Crooks RM. Polymeric self-assembled monolayers. 3. Pattern transfer by use of photolithography, electrochemical methods, and an ultrathin, self-assembled diacetylenic resist. J Am Chem Soc 1995;117:5875-6.
89. Yang W, Ella-Menye JR, Liu S, et al. Cross-linked carboxybetaine SAMs enable nanoparticles with remarkable stability in complex media. Langmuir 2014;30:2522-9.
90. Chien HW, Cheng PH, Chen SY, Yu J, Tsai WB. Low-fouling and functional poly(carboxybetaine) coating via a photo-crosslinking process. Biomater Sci 2017;5:523-31.
91. Wang Y, Sun Y, Ding X, Liang J, Cao X, Tian Z. A combined electro- and photo-chemical approach to repeatedly fabricate two-dimensional molecular assemblies. Electrochimica Acta 2017;246:823-9.
92. Olsen C, Rowntree PA. Bond-selective dissociation of alkanethiol based self-assembled monolayers adsorbed on gold substrates, using low-energy electron beams. The Journal of Chemical Physics 1998;108:3750-64.
93. Zharnikov M, Geyer W, Gölzhäuser A, Frey S, Grunze M. Modification of alkanethiolate monolayers on Au-substrate by low energy electron irradiation: Alkyl chains and the S/Au interface. Phys Chem Chem Phys ;1:3163-71.
94. Zharnikov M, Frey S, Heister K, Grunze M. Modification of alkanethiolate monolayers by low energy electron irradiation: dependence on the substrate material and on the length and isotopic composition of the alkyl chains. Langmuir 2000;16:2697-705.
95. Huels MA, Dugal P, Sanche L. Degradation of functionalized alkanethiolate monolayers by 0-18 eV electrons. The Journal of Chemical Physics 2003;118:11168-78.
96. Waske PA, Meyerbröker N, Eck W, Zharnikov M. Self-assembled monolayers of cyclic aliphatic thiols and their reaction toward electron irradiation. J Phys Chem C 2012;116:13559-68.
97. Saha S, Bruening ML, Baker GL. Facile synthesis of thick films of poly(methyl methacrylate), poly(styrene), and poly(vinyl pyridine) from Au surfaces. ACS Appl Mater Interfaces 2011;3:3042-8.
98. Tkachev M, Anand-kumar T, Bitler A, Guliamov R, Naaman R. Enabling long-term operation of GaAs-based sensors. ENG 2013;05:1-12.
99. Hou T, Greenlief CM, Keller SW, Nelen L, Kauffman JF. Passivation of GaAs (100) with an adhesion promoting self-assembled monolayer. Chem Mater 1997;9:3181-6.
100. Masi G, Balbo A, Esvan J, et al. X-ray photoelectron spectroscopy as a tool to investigate silane-based coatings for the protection of outdoor bronze: the role of alloying elements. Applied Surface Science 2018;433:468-79.
101. Sui W, Zhao W, Zhang X, Peng S, Zeng Z, Xue Q. Comparative anti-corrosion properties of alkylthiols SAMs and mercapto functional silica sol-gel coatings on copper surface in sodium chloride solution. J Sol-Gel Sci Technol 2016;80:567-78.
102. Sharma H, Moumanis K, Dubowski JJ. pH-dependent photocorrosion of GaAs/AlGaAs quantum well microstructures. J Phys Chem C 2016;120:26129-37.
103. Savard S, Blanchard L, Léonard J, Prud’homme RE. Hydrolysis and condensation of silanes in aqueous solutions: hydrolysis and condensation of silanes in aqueous solutions. Polym Compos 1984;5:242-9.
104. Itoh M, Nishihara H, Aramaki K. A chemical modification of alkanethiol Self-assembled monolayers with alkyltrichlorosilanes for the protection of copper against corrosion. J Electrochem Soc 1994;141:2018-23.
107. Green MA, Hishikawa Y, Dunlop ED, Levi DH, Hohl-ebinger J, Ho-baillie AW. Solar cell efficiency tables (version 51). Prog Photovolt Res Appl 2018;26:3-12.
108. Sheldon MT, Eisler CN, Atwater HA. GaAs Passivation with trioctylphosphine sulfide for enhanced solar cell efficiency and durability. Adv Energy Mater 2012;2:339-44.
109. Carpenter MS, Melloch MR, Lundstrom MS, Tobin SP. Effects of Na2S and (NH4)2S edge passivation treatments on the dark current-voltage characteristics of GaAs. pn ;52:2157-9.
110. Sheen CW, Shi JX, Maartensson J, Parikh AN, Allara DL. A new class of organized self-assembled monolayers: alkane thiols on gallium arsenide(100). J Am Chem Soc 1992;114:1514-5.
111. Kirchner C, George M, Stein B, Parak W, Gaub H, Seitz M. Corrosion protection and long-term chemical functionalization of gallium arsenide in an aqueous environment. Adv Funct Mater ;12:266.
112. Carey RI, Folkers JP, Whitesides GM. Self-assembled monolayers containing .omega.-mercaptoalkyl boronic acids adsorbed onto gold form a highly cross-linked, thermally stable borate glass surface. Langmuir 1994;10:2228-34.
113. Fabre B, Hauquier F. Boronic acid-functionalized oxide-free silicon surfaces for the electrochemical sensing of dopamine. Langmuir 2017;33:8693-9.
114. Lenk TJ, Hallmark VM, Hoffmann CL, et al. Structural investigation of molecular organization in self-assembled monolayers of a semifluorinated amidethiol. Langmuir 1994;10:4610-7.
115. Tam-chang S, Biebuyck HA, Whitesides GM, Jeon N, Nuzzo RG. Self-assembled monolayers on gold generated from alkanethiols with the structure RNHCOCH2SH. Langmuir 1995;11:4371-82.
116. Sastry M, Patil V, Mayya KS. Selective binding of divalent cations at the surface of self-assembled monolayers of an aromatic bifunctional molecule studied on a quartz crystal microbalance. J Phys Chem B 1997;101:1167-70.
117. Kim JH, Shin HS, Kim SB, Hasegawa T. Hydrogen-bonding networks of dialkyl disulfides containing the urea moiety in self-assembled monolayers. Langmuir 2004;20:1674-9.
118. Thomas JC, Goronzy DP, Dragomiretskiy K, et al. Mapping buried hydrogen-bonding networks. ACS Nano 2016;10:5446-51.
119. Valiokas R, Östblom M, Svedhem S, Svensson SCT, Liedberg B. Thermal stability of self-assembled monolayers: influence of lateral hydrogen bonding. J Phys Chem B 2002;106:10401-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yu T, Marquez MD, Tran HV, Lee TR. Crosslinked organosulfur-based self-assembled monolayers: formation and applications. Soft Sci 2022;2:5. http://dx.doi.org/10.20517/ss.2022.04
AMA Style
Yu T, Marquez MD, Tran HV, Lee TR. Crosslinked organosulfur-based self-assembled monolayers: formation and applications. Soft Science. 2022; 2(2): 5. http://dx.doi.org/10.20517/ss.2022.04
Chicago/Turabian Style
Yu, Tianlang, Maria D. Marquez, Hung-Vu Tran, T. Randall Lee. 2022. "Crosslinked organosulfur-based self-assembled monolayers: formation and applications" Soft Science. 2, no.2: 5. http://dx.doi.org/10.20517/ss.2022.04
ACS Style
Yu, T.; Marquez MD.; Tran H.V.; Lee TR. Crosslinked organosulfur-based self-assembled monolayers: formation and applications. Soft. Sci. 2022, 2, 5. http://dx.doi.org/10.20517/ss.2022.04
About This Article
Copyright
Data & Comments
Data
 Cite This Article 23 clicks
Cite This Article 23 clicks
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.