Unraveling the main chain effects of fused thiophene conjugated polymers in electrochromism
Abstract
The influence of increasing fused thiophene rings for the corresponding conjugated polymers [polythiophene (PT), poly(thieno[3,2-b]thiophene) (PTT) and poly(dithieno[3,2-b:2’,3’-d]thiophene) (PDTT)] on their photophysical and electrochemical properties, morphology and electrochromic performance are investigated in detail in this study. PDTT is the easiest of the three polymers to prepare and has the lowest onset oxidation potential of 1.17 V because of its increased donor ability, lower than those of PTT (1.41 V) and PT (1.82 V). PDTT also exhibits the best electrochemical and thermal stability because of its extended conjugated skeleton. The PT, PTT and PDTT polymers present poor, good and moderate electrochromic properties, respectively, with increasing fused thiophene rings. PTT displays the highest ΔT of 35% in 700 nm, the fastest response time of 1.0 s and the maximum colouration efficiency (CE) of 94 cm2 C-1, which is attributed to its enhanced morphology, since the PTT film is conducive to the promotion of ions to dope and dedope. Flexible electrochromic devices are fabricated and PTT exhibits the highest ΔT (60% in 480 nm and 16% in 660 nm), as well as excellent stability with less than a 5% ΔT reduction after successive cycling of 1000 s. All these findings indicate that the precise regulation of the fused thiophene is crucial in achieving high performance in electrochromism, which provides insight for the design of electrochromic conjugated polymers and flexible electrochromic devices.
Keywords
INTRODUCTION
Photoelectric properties vary with an increasing number of fused thiophene rings in the order of thiophene (T), thieno[3,2-b]thiophene (TT), dithieno[3,2-b:2’,3’-d]thiophene (DTT) and so on[1,2]. Recently, a variety of fused thiophene small molecules and conjugated polymers have been widely studied because of their extensive conjugation, strong intermolecular S-S interactions and π-π stacking, as well as high ambient stabilities[3-6]. In the field of organic field effect transistors (OFETs), Vegiraju et al.[7] revealed that the introduction of fused thiophene enhanced the neighboring molecular orbital overlapping and enabled more efficient charge carrier transport. Vegiraju et al.[8] reported three new organic semiconductors, which presented an increased maximum hole mobility of 0.81 cm2 V-1 s-1 with a gradual increase in fused thiophene. For organic solar cells (OSCs), Gao et al.[9] demonstrated that multiple fused thiophene-modified asymmetric acceptors could be enabled to achieve an over 17% efficiency via conformation effects on regulating molecular properties. Chen et al.[10] presented an increased aggregation strength as the π-spacer (T, TT and DTT) became larger and generated high-performance thick-film OSCs with 9.1% efficiency. Meanwhile, by enhancing fused thiophene in the main chain to facilitate the carrier transport, thermal stability and π-π stacking interactions, various organic semiconductors have been designed and researched in perovskite solar cells, dye-sensitized solar cells, organic light-emitting diodes, lithium-ion batteries and photocatalytic hydrogen evolution[11-16]. However, comparative studies of fused thiophene organic semiconductors in electrochromism remain relatively unexplored compared to other research areas.
Electrochromic organic semiconductors can easily display visible and reversible changes in transmittance due to the doping-dedoping process. For example, they can change colors by accepting (reduction and dedoping process) or ejecting electrons (oxidation and doping processes) and thus different absorption spectra switching between redox states[17-19]. Electrochromic organic semiconductors have recently become popular due to their excellent processability, outstanding mechanical flexibility, adjustable colors via structural modification, high optical contrast, fast switching properties and superior coloration efficiencies[20-22].
In this contribution, T, TT and DTT monomers [Figure 1] and their corresponding electrochemical polymers, PT, PTT and PDTT, respectively, are comparative studied. Experimental results verify that the main chain configuration change by fused thiophene has complicated effects on their properties, including optical absorption, energy levels, dipole moments, stability, morphology, electrochemistry, electrochromism and potential application in flexible electrochromic devices (ECDs).
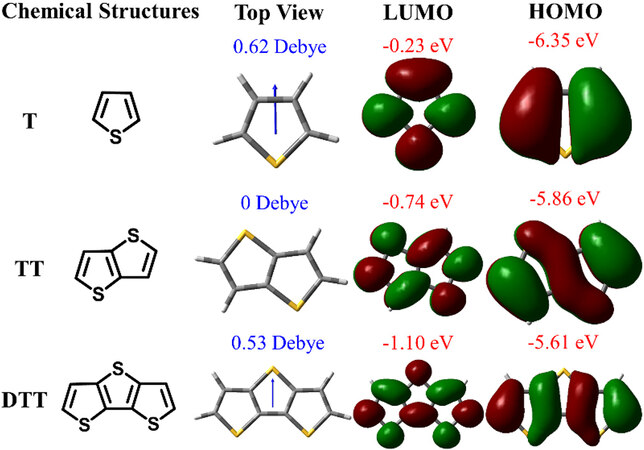
Figure 1. Optimized molecular geometries and frontier molecular orbital distributions of T, TT and DTT using density functional theory by Gaussian 09 at the B3LYP/6-31G(d,p) level. LUMO: Lowest unoccupied molecular orbital; HOMO: highest occupied molecular orbital; T: thiophene; TT: thieno[3,2-b]thiophene; DTT: dithieno[3,2-b:2’,3’-d]thiophene.
EXPERIMENTAL
The experimental part has been given by the author in the Supplementary Material file.
RESULTS AND DISCUSSION
Theoretical calculations
Figure 1 shows the optimized molecular geometries and frontier molecular orbital distributions of T, TT and DTT. All the monomers exhibited complete planar structures with extensive conjugations, which perform with high mobility and promoting crystallization and charge transfer[23]. The high coverages of the lowest unoccupied molecular orbitals (LUMOs) and highest occupied molecular orbitals (HOMOs) were illustrated in the entire conjugated skeletons. The HOMO-LUMO band gaps reduced with increasing fused thiophene rings. TT exhibited a dipole moment of 0 D due to the trans-cis symmetrical sulfur atoms, while T and DTT exhibited dipole moments of 0.62 and 0.53 D, respectively.
Electrochemistry
1H and 13C nuclear magnetic resonance spectral analyses were performed and the chemical structures of T, TT and DTT were confirmed [Supplementary Figures 1-6]. An anodic scan for T, TT and DTT was carried out to explore the effect of molecular structure on the anodic oxidation behavior [Supplementary Figure 7]. The onset oxidation potentials (Eonset) were decreased along with the increase in fused thiophene. The Eonset was 1.82 V for T, 1.41 V for TT and 1.17 V for DTT vs. Ag/AgCl. The superimposed fused thiophene increased the electron density and made their oxidation relatively easy.
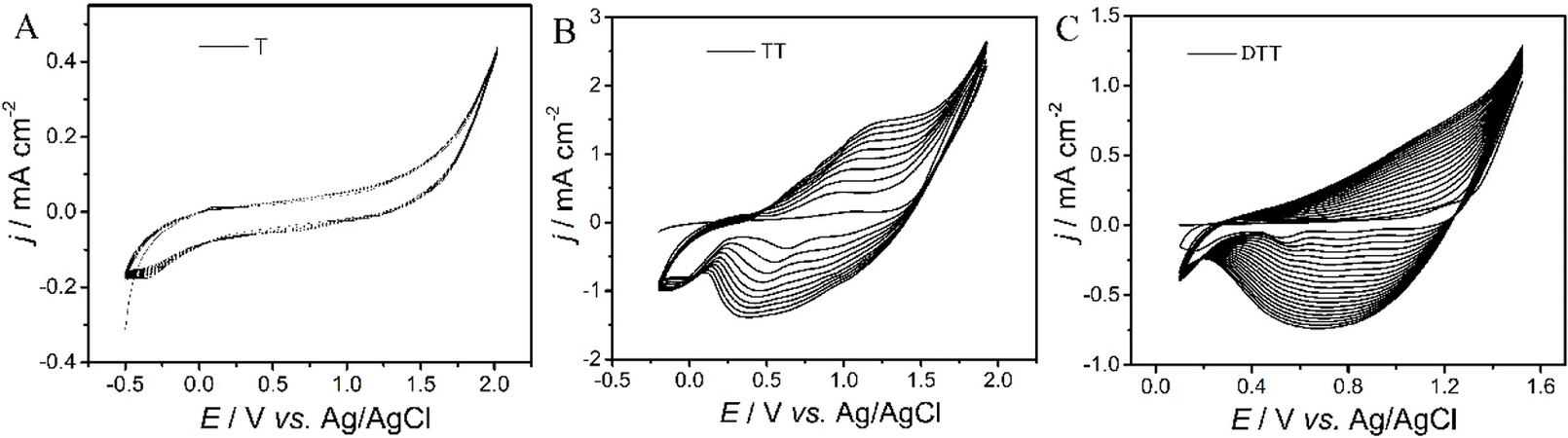
Figure 2A-C show the cyclic voltammograms (CVs) corresponding to the potentiodynamic electropolymerization of T, TT and DTT. TT exhibited obvious broad redox couples in the range of
Figure 2. CVs of 0.01 mol L-1 T (A), TT (B) and DTT (C) in CH2Cl2-Bu4NPF6 (0.1 mol L-1) at a potential scan rate of 100 mV s-1. CVs: Cyclic voltammograms; T: thiophene; TT: thieno[3,2-b]thiophene; DTT: dithieno[3,2-b:2’,3’-d]thiophene.
Similar to the monomers with optimized molecular geometries and frontier molecular orbital distributions, the corresponding polymers (with three repeat units) exhibited completely planar structures, as shown in Supplementary Figure 8. The LUMOs were delocalized mainly on the whole backbone, while the HOMOs were distributed mainly on the core unit. With increasing fused thiophene rings, PT, PTT and PDTT showed gradually deeper LUMO energy levels (-1.70, -2.03 and -2.20 eV, respectively) and raised HOMO energy levels (-5.15, -5.01 and -4.92 eV, respectively). The HOMO-LUMO band gaps of PT, PTT and PDTT were calculated to be 3.45, 2.98 and 2.72 eV, respectively. The results demonstrate that increasing fused thiophene rings have an obvious influence on the energy levels and thus reduce the band gaps.
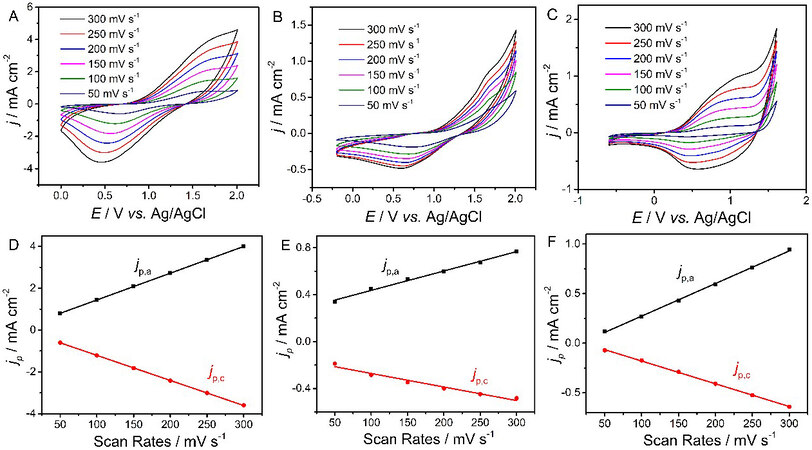
Figure 3A-C illustrate the electrochemical behaviors of PT, PTT and PDTT. All polymers show obvious redox peaks with scarcely any hysteresis (potential drift) along with the reduced scan rates, indicating that all polymers exhibited stable p-doping/dedoping processes at different scan rates from 300 to 50 mV s-1[26,27]. All peak current densities jp,a and jp,c (the anodic and cathodic peak current densities) were proportional to the potential scanning rate (Figure 3D-F, correlation coefficients R2 ≥ 0.99), indicating that the redox process was non-diffusional and electroactive materials were well adhered to the working electrode[18,28]. Redox stability is an evaluation parameter of the electroactive polymers under repeated switching. Herein, long-term CVs of PT, PTT and PDTT were recorded at a scan rate of 200 mV s-1 in CH2Cl2-Bu4NPF6
Figure 3. CVs of PT (A), PTT (B) and PDTT (C) modified Pt electrodes in monomer-free CH2Cl2-Bu4NPF6 (0.10 mol L-1) at potential scan rates of 300-50 mV s-1. Plots of redox peak current densities vs. potential scan rates: PT (D); PTT (E); PDTT (F). jp is the peak current density and jp,a and jp,c denote the anodic and cathodic peak current densities, respectively. CVs: Cyclic voltammograms; PT: polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene).
Optical properties
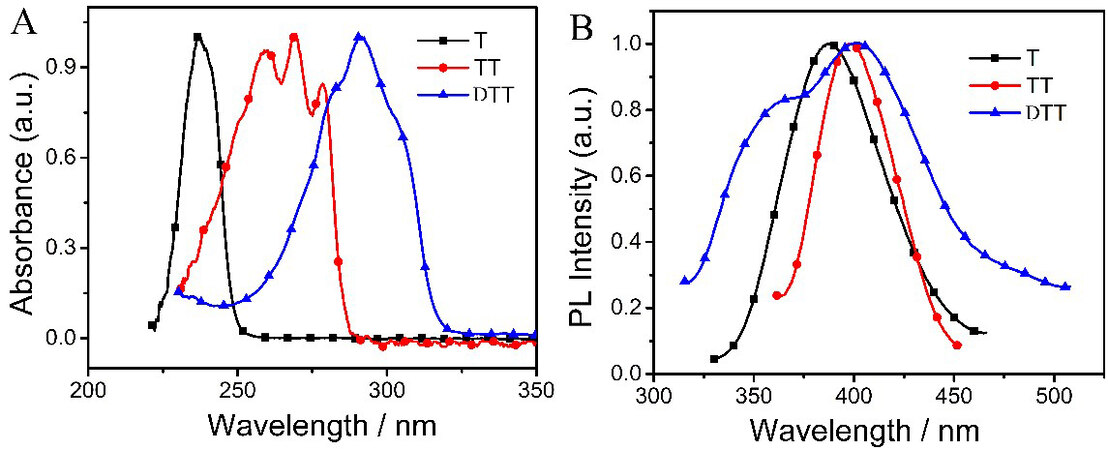
An obvious red shift in the UV-vis spectra was observed with increasing fused thiophene rings in Figure 4A, showing absorption peak centers at 237, 269 and 291 nm, respectively. Interestingly, TT exhibited two obvious sharped shoulder peaks at 260 and 278 nm, while DTT presented inconspicuous broad shoulder peaks. T and TT absorb light significantly in the UV-light region, while DTT absorbs both in the UV-light and visible regions.
Figure 4. UV-vis (A) and fluorescence (B) spectra of T, TT and DTT in CHCl3. T: Thiophene; TT: thieno[3,2-b]thiophene; DTT: dithieno[3,2-b:2’,3’-d]thiophene.
The fluorescence emission spectra of T, TT and DTT are exhibited in Figure 4B. All monomers show maxima at 388, 398 and 402 nm with large Stokes shifts of 151, 129 and 111 nm, respectively, which indicate that they can undergo large conformational changes upon excitation[15].
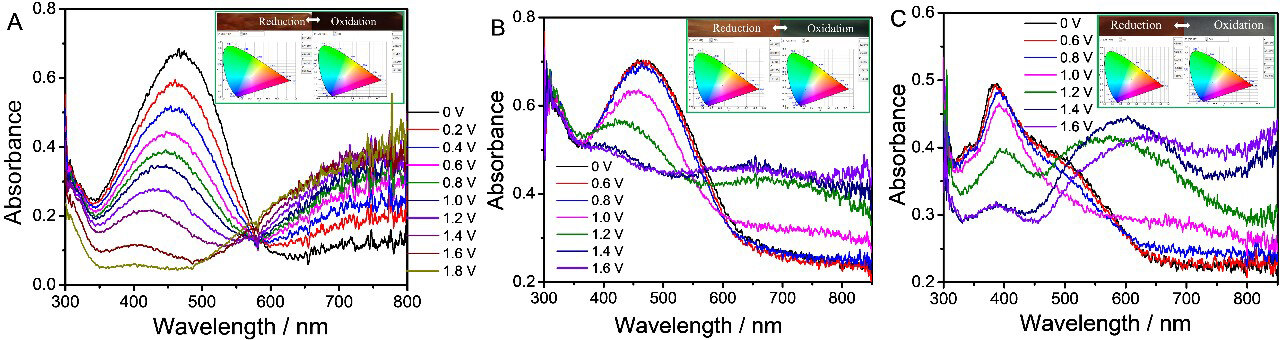
Spectroelectrochemistry
The spectroelectrochemistries of PT (A), PTT (B) and PDTT (C) are given in Figure 5. Taking PDTT as an example, in the reduced form, it shows distinct absorbance peaks centered at 386 nm with two inconspicuous broad shoulder peaks, in accordance with the UV-vis spectrum of DTT. The optical bandgap (Egopt) of PDTT was calculated to be 1.92 eV according to the absorption spectra tailing off to ~647 nm. Upon further oxidation, the absorbance peak of 386 nm underwent a slight red shift and reduction. Meanwhile, two new bands start to intensify at 603 and 900 nm due to the formation of a polaron and bipolaron, respectively[21,22]. In this process, PDTT displayed a distinct color change from dark brown (dedoped: L*: 5.02; a*: -0.85; b*: 3.49) to light blue-green (doped: L*: 5.39; a*: -2.11; b*: -0.72) upon oxidation. The recorded spectra pass through an isosbestic point at ~510 nm, indicating that PDTT was interconverted easily between its neutral and oxidized states[29,30]. In the case of PT and PTT, the color variations were from light brown (dedoped: L*: 6.88; a*: 1.23; b*: 0.87) to dark blue-green (doped: L*: 8.15; a*: -1.27; b*: -4.22) and from moderate brown (dedoped: L*: 7.93; a*: 0.82; b*: 1.91) to moderate blue-green (doped: L*: 10.49; a*:
Figure 5. Spectroelectrochemistry of PT (A), PTT (B) and PDTT (C) on ITO coated glasses in CH3CN-Bu4NPF6, chromaticity diagram (Commission internationale de l'éclairage, CIE 1931) and photograph of polymers in oxidized and reduced states. PT: Polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene); ITO: indium tin oxide.
Electrochromic performance
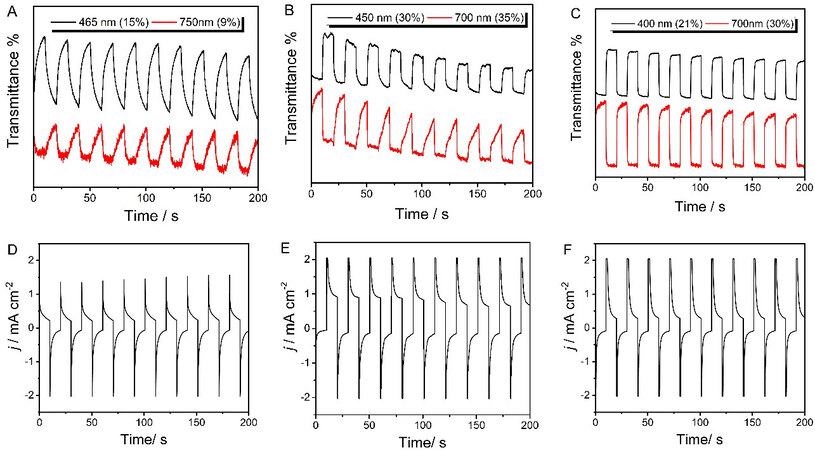
Optical switching studies of the polymer films were measured by a square wave potential step method equipped with optical spectroscopy (chronoabsorptometry) in a monomer-free CH3CN-Bu4NPF6
The time-transmittance curves and corresponding chronoamperometry, as well as other electrochromic parameters, of PT, PTT and PDTT are given in Figure 6 and Table 1. PT, PTT and PDTT exhibited poor, good and moderate electrochromic properties, respectively, with increasing fused thiophene rings. PTT showed the highest ΔT of 35% in 700 nm, the fastest response time of 1.0 s and the maximum CE of
Figure 6. Time-transmittance curves and corresponding chronoamperometry of PT (A, D), PTT (B, E) and PDTT (C, F) in monomer-free CH3CN-Bu4NPF6 (0.1 mol L-1) with an interval of 10 s. PT: Polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene).
Electrochromic parameters of PT, PTT and PDTT
| Sample | Wavelength (nm) | ΔT | Response time (s) | CE (cm2 C-1) | |
| Oxidation | Reduction | ||||
| PT | 465 | 15% | 5.7 | 8.6 | 46 |
| 750 | 9% | 9.2 | 2.5 | 22 | |
| PTT | 450 | 30% | 2.5 | 1.0 | 94 |
| 700 | 35% | 6.0 | 1.1 | 22 | |
| PDTT | 400 | 21% | 1.4 | 1.3 | 18 |
| 600 | 30% | 3.3 | 1.5 | 73 | |
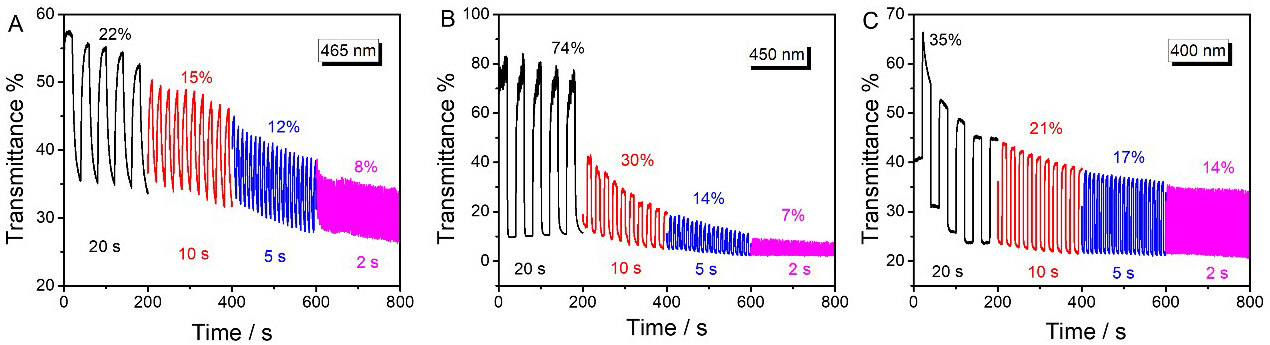
In addition, in order to characterize the influence of different interval times on their electrochromic properties, we also systematically studied the time-transmittance curves of polymers at potential conversion interval times of 20, 10, 5 and 2 s, respectively [Figure 7]. With the decrease of the interval time, the ΔT of the polymers showed an obvious decreasing trend. When the interval time changes from 20 to 2 s, the ΔT of PT at 465 nm decreases from 22% to 8%. For PTT and PDTT, the attenuation values were as high as 91% and 60%, respectively. By comparing these time-transmittance data systematically, we find that a large potential conversion interval time can produce a large ΔT.
Figure 7. Time-transmittance curves of PT (A), PTT (B) and PDTT (C) in monomer-free CH3CN-Bu4NPF6 (0.1 mol L-1) at different intervals. PT: Polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene).
The long-term switching stability (interval time of 20 s) of polymers in the high energy level region was investigated in Supplementary Figure 10. PT exhibited the largest damage from 22% to 3% after 2000 s of switching, which was consistent with the inferior electrochemical stability [Supplementary Figure 8]. The
All polymers switched more rapidly in the reduction process than the oxidation process apart from PT at 465 nm. PTT exhibited the fastest response times of 1.0 and 1.1 s during reduction at both wavelengths, which indicated the highest speed of counter ions moving into and out of the polymer chains during the dedoping processes[21] [Table 1]. PDTT displayed balanced response times of 1.4 and 1.3 s at 400 nm. PT presented the slowest response time.
The CE, as an important parameter, can be calculated as the ratio of the optical density change to the charge injected/rejected per unit. The polymers showed diminishing CE from PTT (94 cm2 C-1) to PDTT
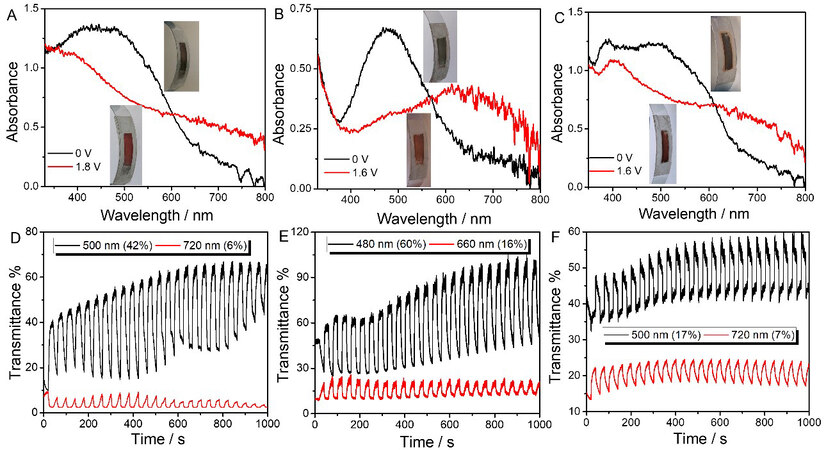
Flexible electrochromic devices
Flexible electrochromic devices were prepared and measured with a device structure of indium tin oxide-polyethylene terephthalate (ITO-PET)/electrochromic active layer (PT, PTT and PDTT)/gel electrolyte (prepared in Supplementary Material)/ITO-PET. As-fabricated flexible ECDs realized reversible color changes between brown and blue-green with an impressive optical contrast [Figure 8A-C], which is in accord with the above non-electrochromic devices. PTT showed the highest ΔT of 60% in 480 nm and 16% in 660 nm, as well as high switching stability with less than 5% optical contrast reduction after successive cycling of 1000 s [Figure 8E]. PT displayed the following high ΔT of 42% in 500 nm (6% in 720 nm) with unstable ΔT changes upon repetitive potential cycling [Figure 8D]. However, PDTT showed the lowest ΔT of 17% in the high energy level region [Figure 8F].
Figure 8. Electrochromic characterization of polymer-based flexible devices. Spectroelectrochemistry and color change of flexible ECDs: PT (A); PTT (B); PDTT (C). Long-term switching stability of such patterned flexible ECDs: PT (D); PTT (E); PDTT (F), with an interval of 20 s. ECDs: Electrochromic devices; PT: polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene).
Morphological and thermal analysis
As shown in Figure 9A-C, the morphologies of the PT, PTT and PDTT polymers were investigated using scanning electron microscopy. PT exhibited phase separation, PTT displayed a smooth surficial morphology and PDTT showed sectional stacking. PTT presented the best morphology, since it was conducive to induce ions to dope and dedope. PT and PDTT films exhibited less long-term switching stability (in Supplementary Figure 10) during the doping and dedoping processes, which is attributed to the agminated domains.
Figure 9. SEM images of PT (A), PTT (B) and PDTT (C) polymerized electrochemically on ITO-coated glass. SEM: Scanning electron microscopy; PT: polythiophene; PTT: poly(thieno[3,2-b]thiophene); PDTT: poly(dithieno[3,2-b:2’,3’-d]thiophene); ITO: indium tin oxide.
Thermogravimetric analytical experiments were performed as shown in Supplementary Figure 11. A small decrease in weight of 2.2% for PT, 0.5% for PT and 0.1% for PDTT below 100 °C was demonstrated to be the evaporation of water. The following weight loss step of 72% for PT, 80% for PTT and only 19% for PDTT were presented at 100-400 °C. When the temperature increased to 800 °C, the residues of PT, PTT and PDTT were 11%, 14% and 49%, respectively. PDTT presented the best thermal stability of all.
CONCLUSIONS
In summary, PT, PTT and PDTT with increasing main chain of fused thiophene, were designed and electrosynthesized. The results show that the conjugated length changes of polymers had an obvious influence on the electrochemical, spectroelectrochemical and electrochromic properties. The onset oxidation potential (Eonset) was decreased from 1.82 to 1.41 and 1.17 V and the spectrum bathochromic shifted along with the increase of fused thiophene. In the reduced state, three polymers displayed a similar color of brown and changed from dark to moderate to light with increasing fused thiophene. In the oxidized state, all polymers showed a similar color of blue-green. PTT displayed a better electrochromic performance than PT and PDTT with the highest ΔT of 35%, the fastest response time of 1.0 s and the maximum CE of
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception: Lin K, Wang Y, Jiang Q
Design of the study: Lin K, Wang Y, Jiang Q
Data analysis and interpretation: Lin K, Liang H, Wu Z, Lu B
Data acquisition: Liang H, Zheng Y, Hu R, Chen H
Administrative, technical, and material support: Zhang X, Xie H
Manuscript writing: Lin K, Jiang Q
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by Guangdong Province Colleges and Universities Young/Characteristic Innovative Talents Project (2019KQNCX190 & 2019KTSCX210), Science and Technology Foundation of Guangdong Province (2021A0101180005), Innovation Team of Colleges and Universities in Guangdong Province (2020KCXTD030), Special Projects in Key Areas for the Universities of Guangdong Province (2020ZDZX2027, 2021ZDZX1009), Jiangxi Key Laboratory of Flexible Electronics (20212BCD42004), Zhongshan Science and Technology Public Project (2020B2027, 2019B2016), and PhD Early Development Program of University of Electronic Science and Technology of China Zhongshan Institute (419YKQN16).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
Supplementary Materials
REFERENCES
1. Cinar ME, Ozturk T. Thienothiophenes, dithienothiophenes, and thienoacenes: syntheses, oligomers, polymers, and properties. Chem Rev 2015;115:3036-140.
2. Xue Z, Chen S, Gao N, et al. Structural design and applications of stereoregular fused thiophenes and their oligomers and polymers. Polymer Reviews 2020;60:318-58.
3. Vegiraju S, Luo X, Li L, et al. Solution processable pseudo n-thienoacenes via intramolecular S···S lock for high performance organic field effect transistors. Chem Mater 2020;32:1422-9.
4. Chen T, Peng K, Lin Y, et al. A chlorinated nonacyclic carbazole-based acceptor affords over 15% efficiency in organic solar cells. J Mater Chem A 2020;8:1131-7.
5. Strakova K, Assies L, Goujon A, Piazzolla F, Humeniuk HV, Matile S. Dithienothiophenes at work: access to mechanosensitive fluorescent probes, chalcogen-bonding catalysis, and beyond. Chem Rev 2019;119:10977-1005.
6. Huang Y, Huang W, Yang J, et al. The synthesis, characterization and flexible OFET application of three (Z)-1,2-bis(4-(tert-butyl)phenyl)ethane based copolymers. Polym Chem 2016;7:538-45.
7. Vegiraju S, He G, Kim C, et al. Solution-processable dithienothiophenoquinoid (DTTQ) structures for ambient-stable n-channel organic field effect transistors. Adv Funct Mater 2017;27:1606761.
8. Vegiraju S, Huang DY, Priyanka P, et al. High performance solution-processable tetrathienoacene (TTAR) based small molecules for organic field effect transistors (OFETs). Chem Commun (Camb) 2017;53:5898-901.
9. Gao W, Fu H, Li Y, et al. Asymmetric acceptors enabling organic solar cells to achieve an over 17% efficiency: conformation effects on regulating molecular properties and suppressing nonradiative energy loss. Adv Energy Mater 2021;11:2003177.
10. Chen P, Shi S, Wang H, et al. Aggregation strength tuning in difluorobenzoxadiazole-based polymeric semiconductors for high-performance thick-film polymer solar cells. ACS Appl Mater Interfaces 2018;10:21481-91.
11. Wang L, Zhuang Q, You G, et al. Donor-acceptor type polymers containing fused-ring units as dopant-free, hole-transporting materials for high-performance perovskite solar cells. ACS Appl Energy Mater 2020;3:12475-83.
12. Liu X, Kong F, Guo F, et al. Influence of π-linker on triphenylamine-based hole transporting materials in perovskite solar cells. Dyes and Pigments 2017;139:129-35.
13. Lu Z, Peng J, Wu A, et al. Heteroleptic ruthenium sensitizers with hydrophobic fused-thiophenes for use in efficient dye-sensitized solar cells. Eur J Inorg Chem 2016;2016:1214-24.
14. Isci R, Tekin E, Kaya K, Piravadili Mucur S, Gorkem SF, Ozturk T. Tetraphenylethylene substituted thienothiophene and dithienothiophene derivatives: synthesis, optical properties and OLED applications. J Mater Chem C 2020;8:7908-15.
15. Xue X, Luo J, Kong L, et al. The synthesis of triazine-thiophene-thiophene conjugated porous polymers and their composites with carbon as anode materials in lithium-ion batteries. RSC Adv 2021;11:10688-98.
16. Cheng J, Tan Z, Xing Y, et al. Exfoliated conjugated porous polymer nanosheets for highly efficient photocatalytic hydrogen evolution. J Mater Chem A 2021;9:5787-95.
17. Monk P, Mortimer R, Rosseinsky D. Electrochromism and electrochromic devices. Cambridge: Cambridge University Press; 2007. p. 512.
18. Beaujuge PM, Reynolds JR. Color control in pi-conjugated organic polymers for use in electrochromic devices. Chem Rev 2010;110:268-320.
19. Gunbas G, Toppare L. Electrochromic conjugated polyheterocycles and derivatives--highlights from the last decade towards realization of long lived aspirations. Chem Commun (Camb) 2012;48:1083-101.
20. Mortimer RJ, Rosseinsky DR, Monk PMS. Electrochromic materials and devices. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2015.
21. Lv X, Li W, Ouyang M, Zhang Y, Wright DS, Zhang C. Polymeric electrochromic materials with donor-acceptor structures. J Mater Chem C 2017;5:12-28.
22. Lin K, Chen S, Lu B, Xu J. Hybrid π-conjugated polymers from dibenzo pentacyclic centers: precursor design, electrosynthesis and electrochromics. Sci China Chem 2017;60:38-53.
23. Liu Y, Song J, Bo Z. Designing high performance conjugated materials for photovoltaic cells with the aid of intramolecular noncovalent interactions. Chem Commun (Camb) 2021;57:302-14.
24. Lu B, Jian N, Qu K, et al. Stepwise enhancement on optoelectronic performances of polyselenophene via electropolymerization of mono-, bi-, and tri-selenophene. Electrochimica Acta 2020;340:135974.
25. Chen W, Xue G. Low potential electrochemical syntheses of heteroaromatic conducting polymers in a novel solvent system based on trifluroborate-ethyl ether. Prog Polym Sci 2005;30:783-811.
26. Inzelt G, Pineri M, Schultze J, Vorotyntsev M. Electron and proton conducting polymers: recent developments and prospects. Electrochimica Acta 2000;45:2403-21.
27. Vorotyntsev M, Badiali J. Short-range electron-ion interaction effects in charging the electroactive polymer films. Electrochimica Acta 1994;39:289-306.
28. Lin K, Ming S, Zhen S, Zhao Y, Lu B, Xu J. Molecular design of DBT/DBF hybrid thiophenes π-conjugated systems and comparative study of their electropolymerization and optoelectronic properties: from comonomers to electrochromic polymers. Polym Chem 2015;6:4575-87.
29. Lin K, Li C, Tao W, et al. Electrochemical synthesis and electro-optical properties of dibenzothiophene/thiophene conjugated polymers with stepwise enhanced conjugation lengths. Front Chem 2020;8:819.
30. Ming S, Zhen S, Lin K, Zhao L, Xu J, Lu B. Thiadiazolo[3,4-c]pyridine as an acceptor toward fast-switching green donor-acceptor-type electrochromic polymer with low bandgap. ACS Appl Mater Interfaces 2015;7:11089-98.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lin K, Liang H, Zheng Y, Hu R, Chen H, Wu Z, Zhang X, Xie H, Wang Y, Jiang Q, Lu B. Unraveling the main chain effects of fused thiophene conjugated polymers in electrochromism. Soft Sci 2021;1:12. http://dx.doi.org/10.20517/ss.2021.15
AMA Style
Lin K, Liang H, Zheng Y, Hu R, Chen H, Wu Z, Zhang X, Xie H, Wang Y, Jiang Q, Lu B. Unraveling the main chain effects of fused thiophene conjugated polymers in electrochromism. Soft Science. 2021; 1(3): 12. http://dx.doi.org/10.20517/ss.2021.15
Chicago/Turabian Style
Lin, Kaiwen, Haoshen Liang, Yawen Zheng, Ronglin Hu, Hong Chen, Zhixin Wu, Xiaobin Zhang, Hui Xie, Yuehui Wang, Qinglin Jiang, Baoyang Lu. 2021. "Unraveling the main chain effects of fused thiophene conjugated polymers in electrochromism" Soft Science. 1, no.3: 12. http://dx.doi.org/10.20517/ss.2021.15
ACS Style
Lin, K.; Liang H.; Zheng Y.; Hu R.; Chen H.; Wu Z.; Zhang X.; Xie H.; Wang Y.; Jiang Q.; Lu B. Unraveling the main chain effects of fused thiophene conjugated polymers in electrochromism. Soft. Sci. 2021, 1, 12. http://dx.doi.org/10.20517/ss.2021.15
About This Article
Copyright
Data & Comments
Data
 Cite This Article 18 clicks
Cite This Article 18 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.